AC Immune and Takeda Strike Exclusive Licensing Deal on Alzheimer’s Amyloid Beta Immunotherapy
Takeda and AC Immune SA have entered into a global exclusive option and licensing deal concerning AC Immune’s active immunotherapies, which are aimed at combating toxic amyloid beta variants, such as ACI-24.060, in the treatment of Alzheimer’s disease.
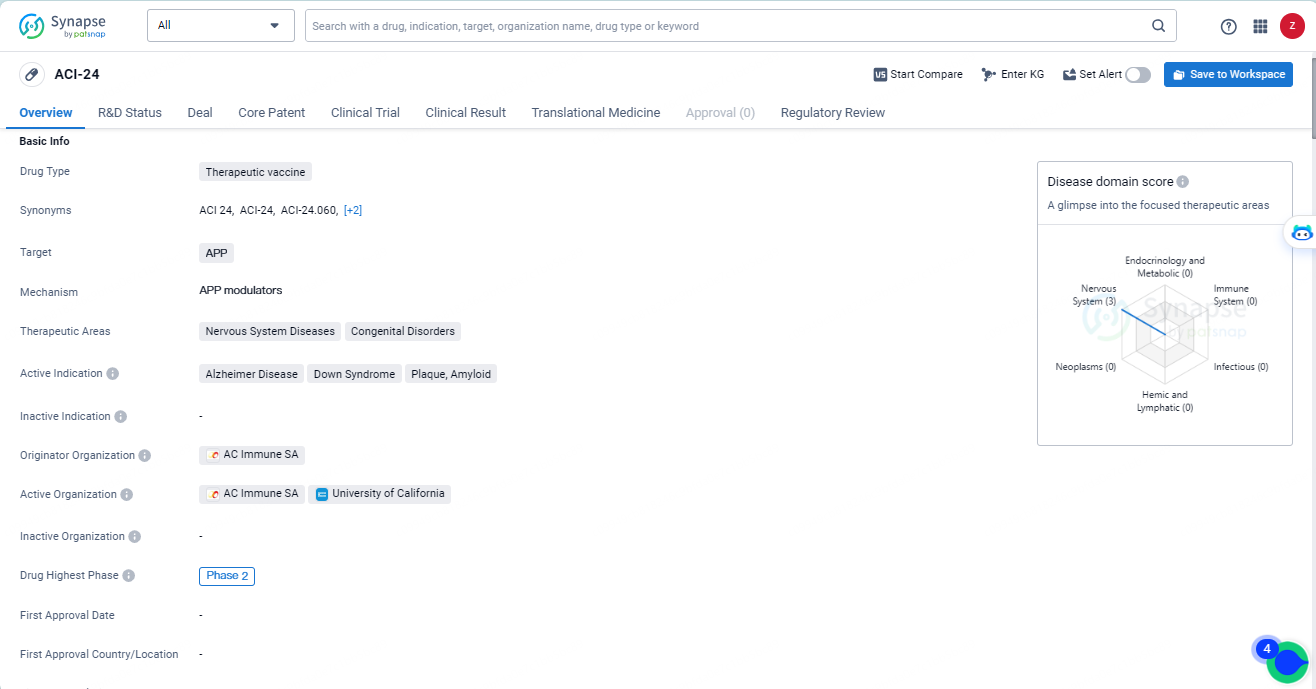
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
ACI-24.060 is a candidate for anti-Abeta active immunotherapy, engineered to elicit a strong antibody response to combat the toxic variants of Abeta, which are thought to contribute to the development of plaques and progression of Alzheimer's disease. The therapy aims to both diminish existing plaques and prevent new plaques from forming in the brain, potentially decelerating or arresting the advancement of Alzheimer's disease.
Currently, ACI-24.060 is under examination in the ABATE study—a randomized, double-blind, placebo-controlled Phase 1b/2 trial. This study is designed to evaluate its safety, tolerability, immunogenic response, and pharmacodynamic impacts in individuals with early-stage Alzheimer's disease and in adults diagnosed with Down syndrome.
"Leading the forefront in active immunotherapy, our team is crafting a groundbreaking strategy poised to redefine how Alzheimer's disease is treated and to lessen the extensive challenge faced by patients and society," commented Dr. Andrea Pfeifer, CEO of AC Immune. "In this pivotal phase of development, collaborating with Takeda enhances our ability to proceed into Phase 3 trials swiftly," she asserted.
Dr. Pfeifer further stated, “This partnership benefits from Takeda's operational excellence, strategic acumen and financial resources, fortifying our capabilities to conduct global Phase 3 studies effectively. This arrangement also fosters our focus to finalize Phase 1b/2 trials and boost our initiatives in advancing our preliminary pipeline with better funding.”
According to the collaboration terms, AC Immune will initially receive $100 million and might gain additional payments including an option exercise fee, along with development, commercial, and sales milestones, potentially totaling up to about $2.1 billion if all milestones are met during the agreement's term. When the product hits the market, AC Immune will earn tiered double-digit royalties based on global net sales.
Extra specifics of the deal can be found in the Form 6-K submitted by AC Immune today to the U.S. Securities and Exchange Commission. The effectiveness of Takeda’s license after the option exercise will depend on the completion or expiry of the waiting periods mandated by the Hart-Scott-Rodino Act.
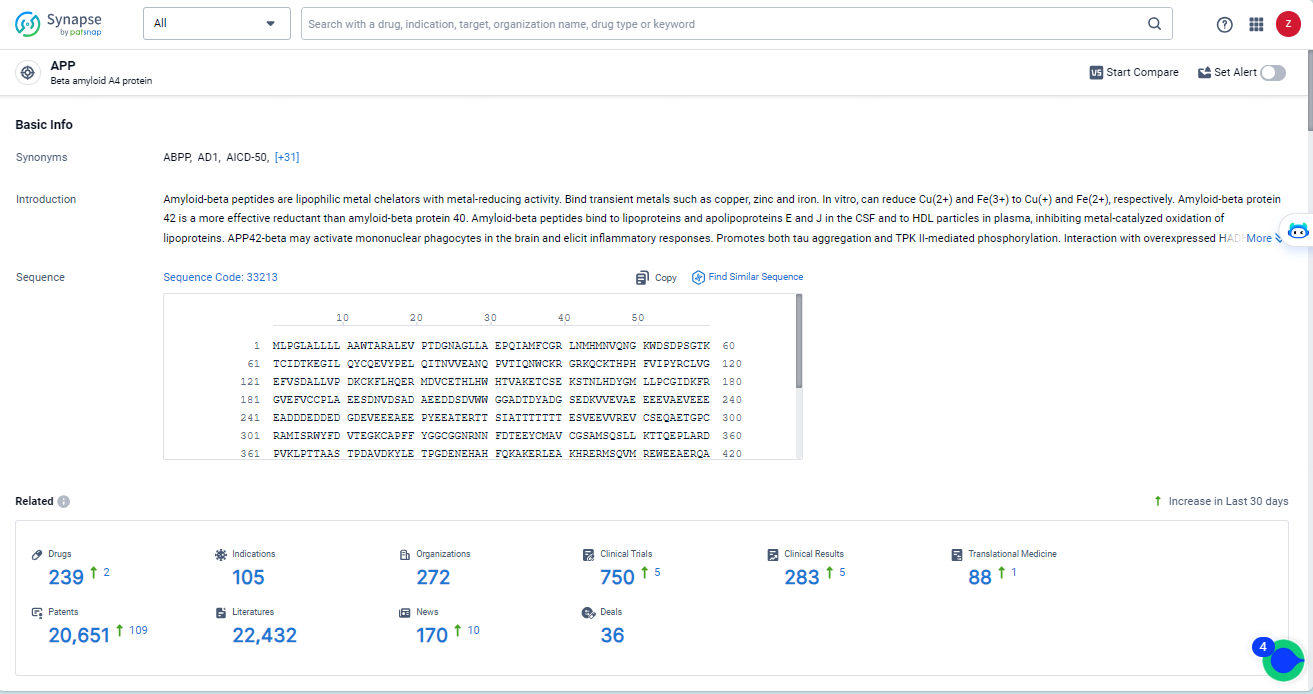
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 239 investigational drugs for the APP targets, including 105 indications, 272 R&D institutions involved, with related clinical trials reaching 750, and as many as 20651 patents.
ACI-24 is a therapeutic vaccine being developed by AC Immune SA for the treatment of nervous system diseases and congenital disorders. The drug targets APP and is currently in Phase 2 of clinical development. With its Fast Track designation, ACI-24 has the potential to address unmet medical needs in conditions such as Alzheimer's disease and Down syndrome.