Promising Phase 2a Results for Cardurion's Heart Failure Treatment
Cardurion Pharmaceuticals, Inc., an enterprise in the clinical phase of biotech focused on creating advanced therapeutic solutions for cardiovascular conditions, disclosed the release of encouraging clinical outcomes from its CARDINAL‑HF study. This marks the initial Phase 2 proof-of-concept trial for a phosphodiesterase-9 (PDE9) inhibitor involving heart failure patients.
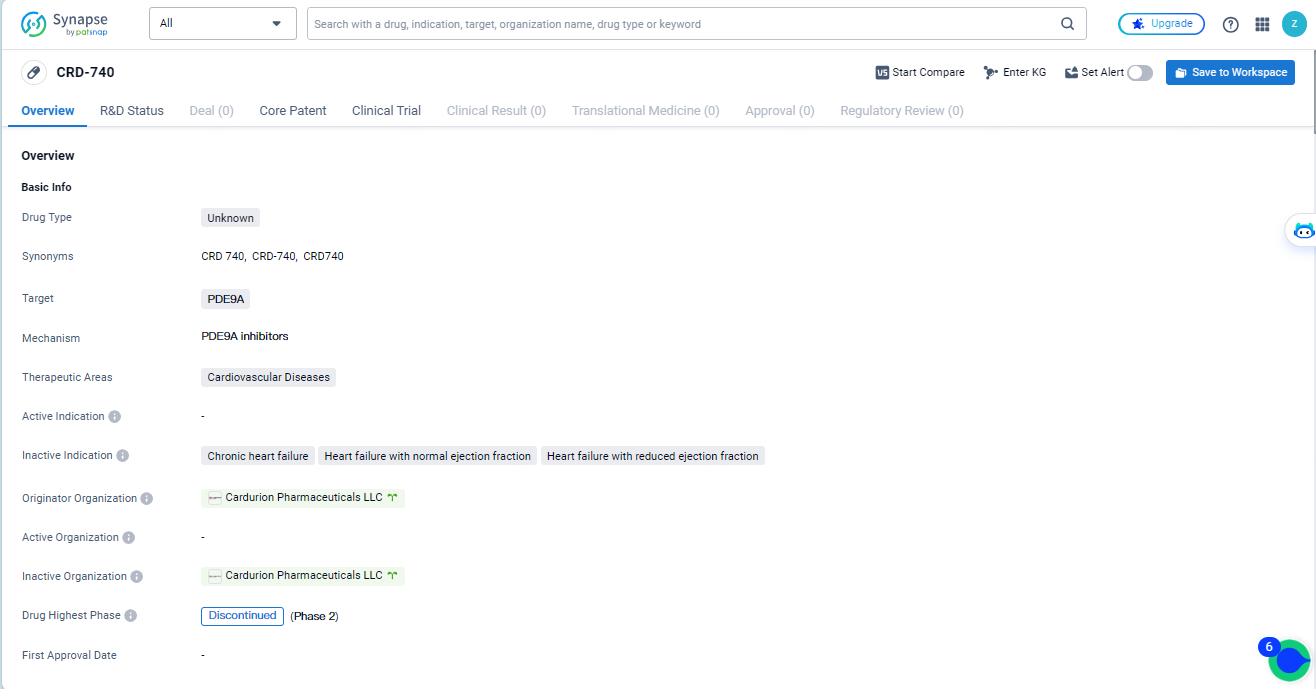
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the CARDINAL-HF Phase 2a trial, CRD-740 successfully met its primary goal among patients suffering from heart failure with reduced ejection fraction, showing a significant statistical rise in the median level of plasma cyclic guanosine monophosphate following a treatment duration of four weeks.
Plasma cGMP serves as a key indicator for intracellular cGMP. The levels of this biomarker mirror the functioning of the cardioprotective natriuretic peptide signaling pathway, recognized for its therapeutic advantages in managing heart failure. Throughout the trial, CRD-740 showed a high level of tolerability.
James Udelson, MD, the Chief of Cardiology at Tufts Medical Center and the Principal Investigator of the CARDINAL-HF trial, commented, “The encouraging outcomes from this initial Phase 2 proof-of-concept study of a PDE9 inhibitor in heart failure cases mark a crucial advancement in exploring this innovative approach.”
Scott D. Solomon, MD, a Professor of Medicine at Harvard Medical School remarked, “The strategy of targeting the NP signaling pathway follows current standard treatments for heart failure. Introducing PDE9 inhibition offers a fresh method to activate this pathway, aiming to enhance patient results. Heart failure remains a widespread and increasing chronic issue, which continues to significantly impact the well-being and survival of countless individuals.”
The trial recorded that CRD-740 was well received among participants with HFrEF, representing roughly fifty percent of individuals dealing with chronic heart failure. Inhibition of PDE9 by CRD-740 led to a significant median rise in plasma cGMP levels after four weeks as opposed to a placebo, achieving the trial's primary outcome. Moreover, notable median increases in urinary cGMP levels were also noted in those treated with CRD-740 compared with those who received a placebo.
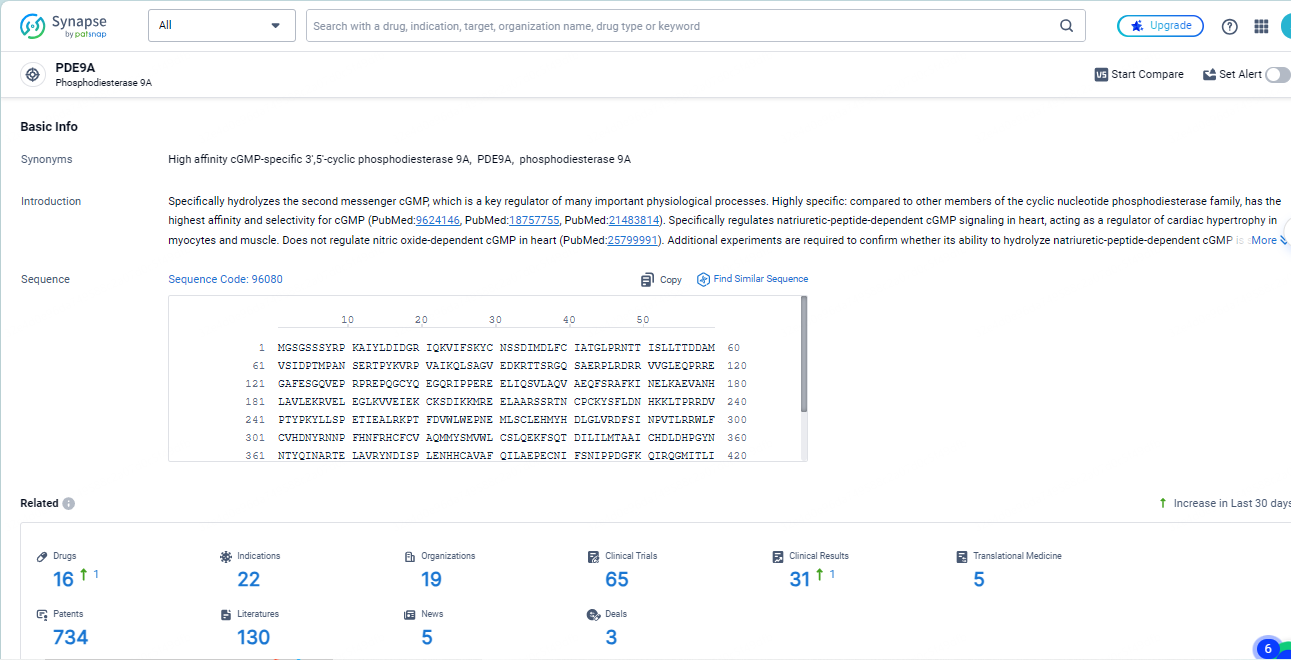
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 16 investigational drugs for the PDE9A targets, including 22 indications, 19 R&D institutions involved, with related clinical trials reaching 65, and as many as 734 patents.
CRD-740 targets PDE9A and is intended for use in cardiovascular diseases. Developed by Cardurion Pharmaceuticals LLC, the drug's development has been discontinued, indicating that it did not progress beyond a certain phase of development. Further information is needed to evaluate the drug's potential benefits and limitations in treating cardiovascular diseases.