Adcentrx Therapeutics starts Phase 1a/b trials of ADRX-0706, a new ADC targeting Nectin-4, for advanced solid tumors
A biotech firm named Adcentrx Therapeutics, committed to innovating Antibody-Drug Conjugate therapies for cancer and serious diseases, has confirmed the initial dosage administered to a patient in the Phase 1a/b trial of ADRX-0706, aimed at treating advanced solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are excited to signal this significant turning point for Adcentrx, changing our status from a discovery-based entity to a clinical-stage organization," commented Hui Li, Ph.D., the Initiator and CEO of Adcentrx. "We are highly hopeful about the promise of ADRX-0706 to cater to the undiscovered demands of patients dealing with Nectin-4 expressing cancers."
The pioneering Phase 1a/b clinical trial for ADRX-0706 is a public-notice, multiple center dosage expansion and escalation study. Currently, it is classifying patients with chosen advanced solid tumors. Optimum dosage determination of ADRX-0706, as well as its safety and tolerance parameters, are the focus of this research. Preliminary data from this study is expected by mid-2024.
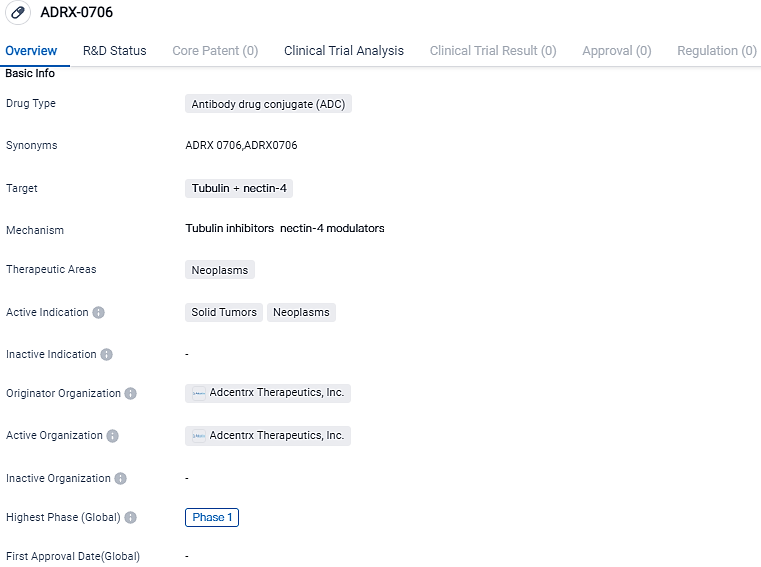
ADRX-0706 is an ADC product candidate identified by Adcentrx. The antibody element of this product is aimed at Nectin-4, a cell membrane adhesion protein which is over-represented in several human cancers and links with ill-favored disease prognosis.
Applying Adcentrx's owned i-Conjugation™ technology and unique tubulin inhibitor payload, AP052, an ADC with a drug-antibody ratio of eight is generated - ADRX-0706. In preclinical models, ADRX-0706 shows a beneficial safety and pharmacokinetic profile and significant effectiveness across diverse tumor implications.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
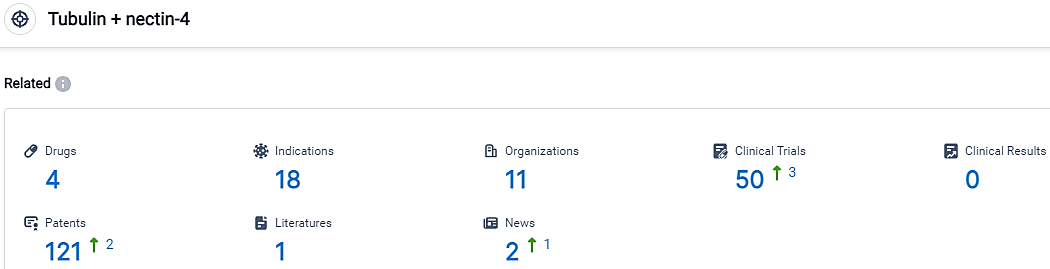
According to the data provided by the Synapse Database, As of October 5, 2023, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 18 indications,11 R&D institutions involved, with related clinical trials reaching 50,and as many as 121 patents.
The medical compound ADRX-0706 is a type of antibody drug conjugate, devised to target tubulin and nectin-4 proteins to cater neoplasms, particularly solid tumors. The compound is presently in the preliminary stage, Phase 1, of clinical testing and is a developmental undertaking by Adcentrx Therapeutics, Inc. Further investigation and clinical experiments will be indispensable to assess the efficacy and probable advantages of ADRX-0706 for cancer treatment.