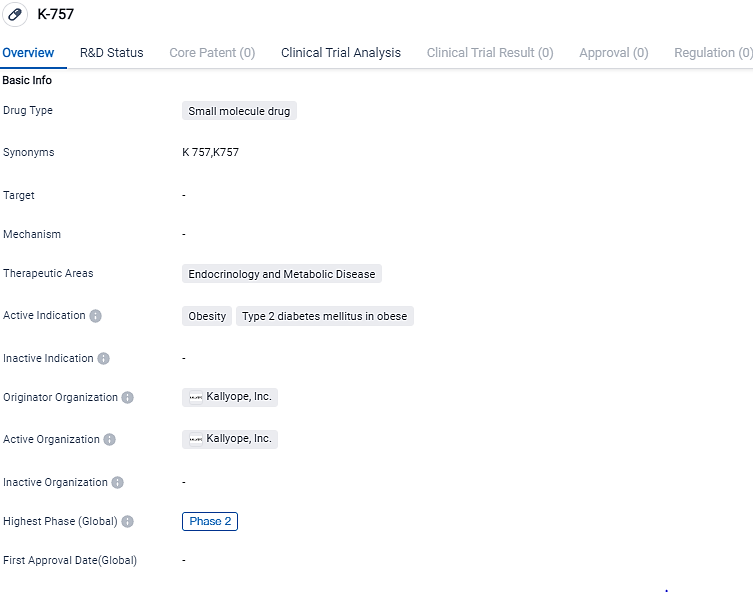
Kallyope progresses to Phase 2, pioneering an oral treatment strategy for both obesity and Type 2 Diabetes
Kallyope, Inc., a biotech firm in clinical phase focusing on the identification and creation of innovative, orally-consumed, small-molecule treatments for conditions with significant unmet demand, has commenced its Phase 2 experiment to assess K-757 and K-833. These innovative oral nutrient receptor stimulants are being examined for their potential in combating obesity and Type 2 diabetes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The purpose of the K-757 P006 trial is to establish the effectiveness, safety, and patient acceptance of these new treatments for obesity, a serious health issue that is becoming more prevalent in the United States. Losing weight can greatly contribute towards managing severe health risks such as Type 2 diabetes, cardiovascular disease, and elevated cholesterol.
Although modern medical treatments are available for obesity and diabetes, only 2 percent of U.S. obesity patients are treated medically. Wide access to helpful, user-friendly, and easily tolerated oral medicines will be a crucial move in attaining this underserved necessity more inclusively.
The immediate need for oral, user-friendly, more tolerable, and cost-effective solutions to address the significant unmet requirements for those suffering from obesity is evident," stated Jay Galeota, Kallyope's president and CEO. "K-757 and K-833 introduce an unprecedented oral strategy that could significantly improve treatment possibilities for those affected by obesity and Type 2 diabetes.”
"It is essential to discover new treatments that are safe, well-accepted, and effective for losing weight, either as solo drugs or in conjunction with others," Brett Lauring, M.D., Ph.D., Chief Medical Officer of Kallyope said. “The community of obesity patients is extremely diverse and is just starting to be studied, and there is a need for a variety of treatments from differing classes to address their unique situations.”
“The Kallyope team has made significant headway in moving these pioneering substances from research to clinical trials,” shared Nancy Thornberry, founding CEO and head of Research and Development at Kallyope. “K-757 and K-833’s mode of action is fundamentally unlike the GLP-1 agonists currently available. This approach is expected to be beneficial for weight loss and blood sugar regulation and might be easier to administer and accept than GLP-1s.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
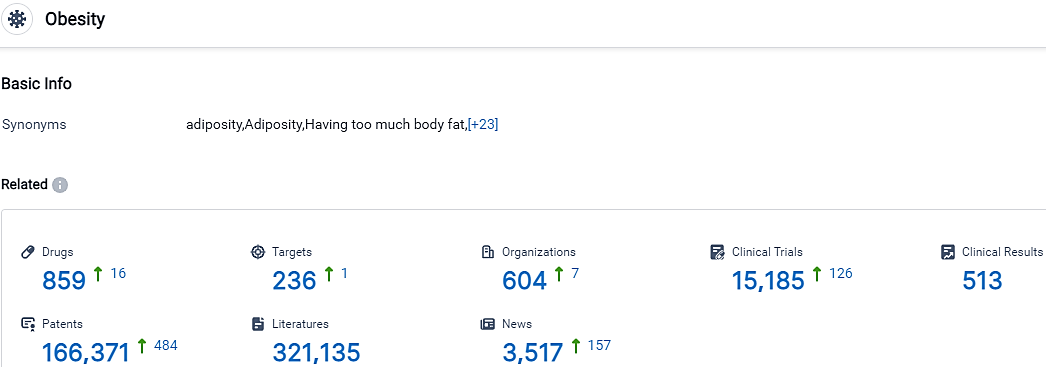
According to the data provided by the Synapse Database, As of October 5, 2023, there are 859 investigational drugs for the Obesity, including 236 targets, 604 R&D institutions involved, with related clinical trials reaching 15185,and as many as 166371 patents.
K-757 and K-833 serve as oral nutrient receptor stimulants that intensify the body's innate metabolic messages to provoke the production of several hunger-controlling hormones like GLP-1, along with various other scientifically confirmed hormones such as PYY and CCK. The impact this has on these naturally occurring hunger-controlling hormones liken to what is observed post bariatric surgery. These are currently the sole recognized oral nutrient receptor stimulants under research for managing obesity.