Advancements in Clinical Research on CDK5 Inhibitors
CDK5, or cyclin-dependent kinase 5, is a crucial enzyme found in the human body that plays a significant role in various physiological processes. It is primarily involved in the development and maintenance of the central nervous system, including neuronal migration, synaptic plasticity, and neurotransmitter release. CDK5 also regulates cell cycle progression, neuronal survival, and differentiation. Dysregulation of CDK5 has been implicated in several neurological disorders, such as Alzheimer's disease, Parkinson's disease, and epilepsy. Understanding the role of CDK5 in these conditions can provide valuable insights for the development of targeted therapies and interventions to treat and manage these disorders.
CDK5 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 8 CDK5 drugs worldwide, from 18 organizations, covering 24 indications, and conducting 19 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.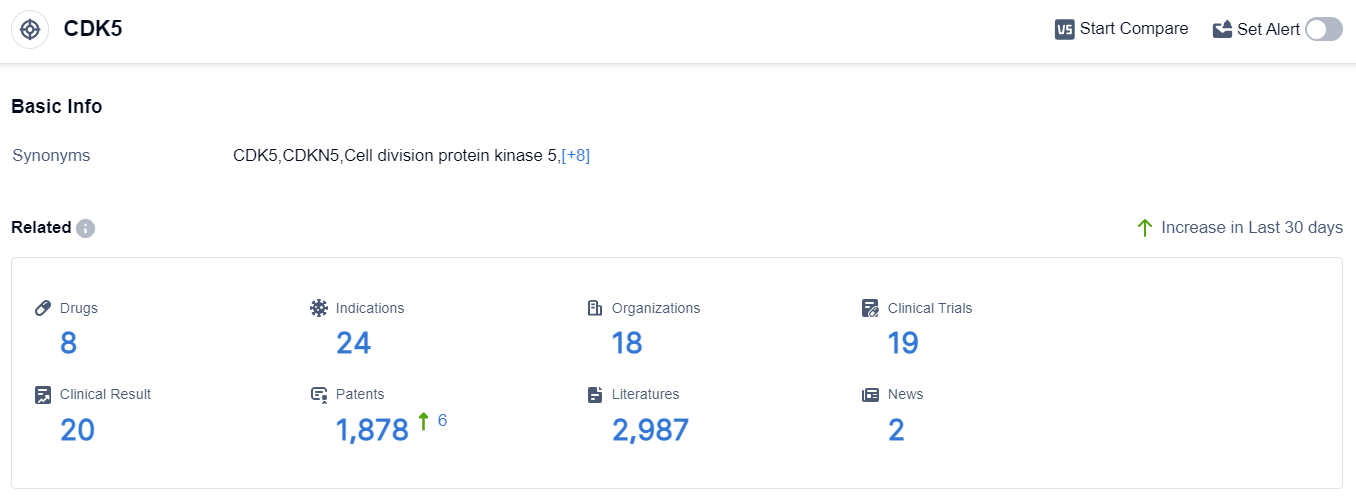
The current competitive landscape of target CDK5 indicates a strong focus on its development by companies such as Merck KGaA, AbbVie, Inc., and Cothera Bioscience, Inc. Merck KGaA leads the way with drugs in Phase 3 and the inactive stage.
Indications being targeted include various types of cancers and neurological disorders. Small molecule drugs are progressing rapidly, indicating a focus on developing orally available drugs with high specificity for CDK5.
The United States and the European Union are the leading countries/locations in the development of CDK5 inhibitors. Overall, the future development of target CDK5 shows promising potential for the treatment of various diseases, but further research and clinical trials are needed to bring these drugs to market.
The multi-target CDK5 inhibitor ZTR enters Phase I clinical trials
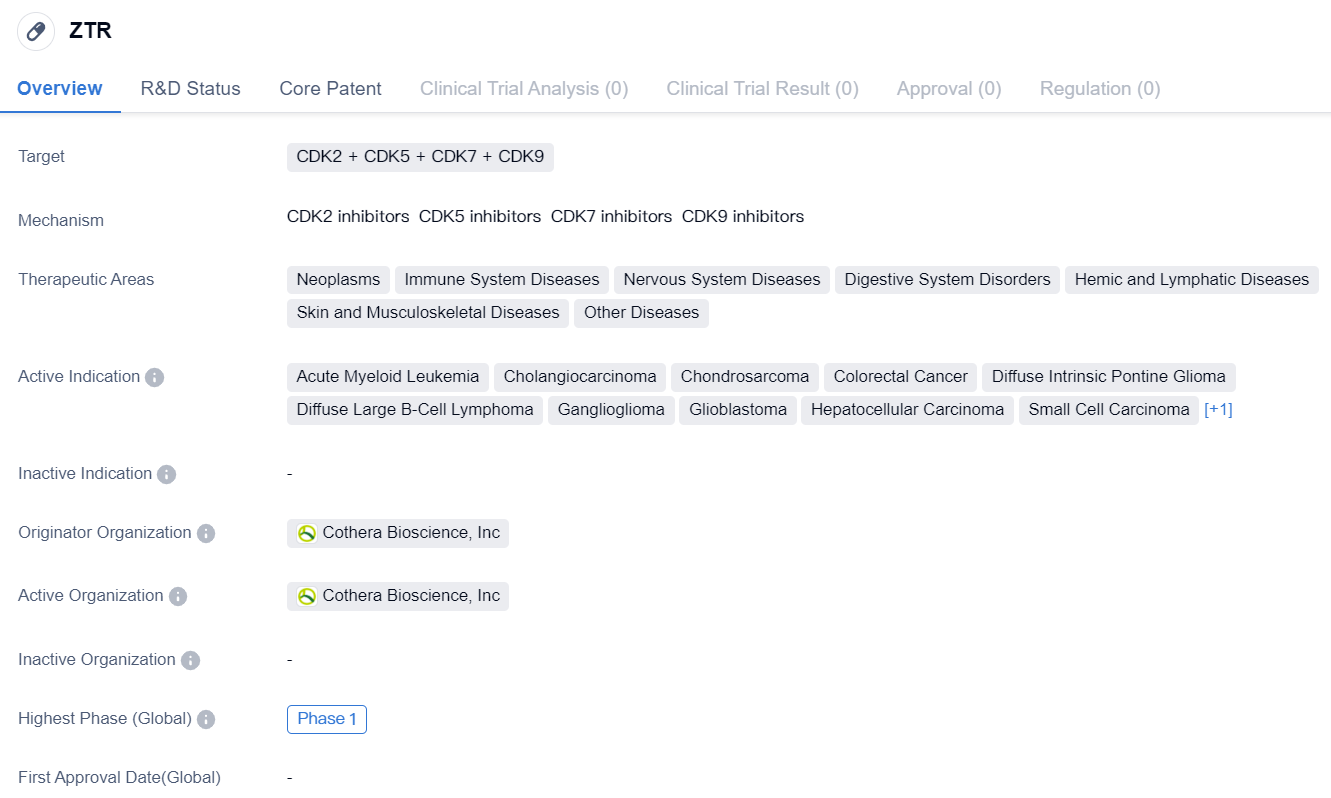
The drug ZTR is a small molecule drug that targets CDK2, CDK5, CDK7, and CDK9. It falls under the therapeutic areas of neoplasms, immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, and other diseases. ZTR is indicated for the treatment of various types of cancers including acute myeloid leukemia, cholangiocarcinoma, chondrosarcoma, colorectal cancer, diffuse intrinsic pontine glioma, diffuse large B-cell lymphoma, ganglioglioma, glioblastoma, hepatocellular carcinoma, small cell carcinoma, and triple negative breast cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is developed by Cothera Bioscience, Inc, which is the originator organization. Currently, ZTR is in Phase 1. This indicates that the drug is still in the early stages of clinical trials and further research and testing are required before it can be approved for commercial use.
ZTR's targeting of CDK2, CDK5, CDK7, and CDK9 suggests that it may have potential in inhibiting the growth and proliferation of cancer cells. CDKs, or cyclin-dependent kinases, are enzymes that play a crucial role in cell cycle regulation. By targeting multiple CDKs, ZTR may have a broader spectrum of activity against different types of cancers.
The therapeutic areas of ZTR cover a wide range of diseases, indicating its potential for treating various medical conditions. Neoplasms, immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, and skin and musculoskeletal diseases are all areas where ZTR may have therapeutic benefits. This suggests that the drug may have a versatile mechanism of action that could be applicable to different disease pathways.
However, it is important to note that the information provided is limited to the objective facts about ZTR. Further research and clinical trials are needed to determine the drug's efficacy, safety profile, and potential side effects. The current phase of development, Phase 1, indicates that ZTR is still in the early stages of evaluation and it will be some time before it can progress to later phases and potentially be approved for commercial use.
The selective CDK5 inhibitor MRT3-007 is in the preclinical research stage
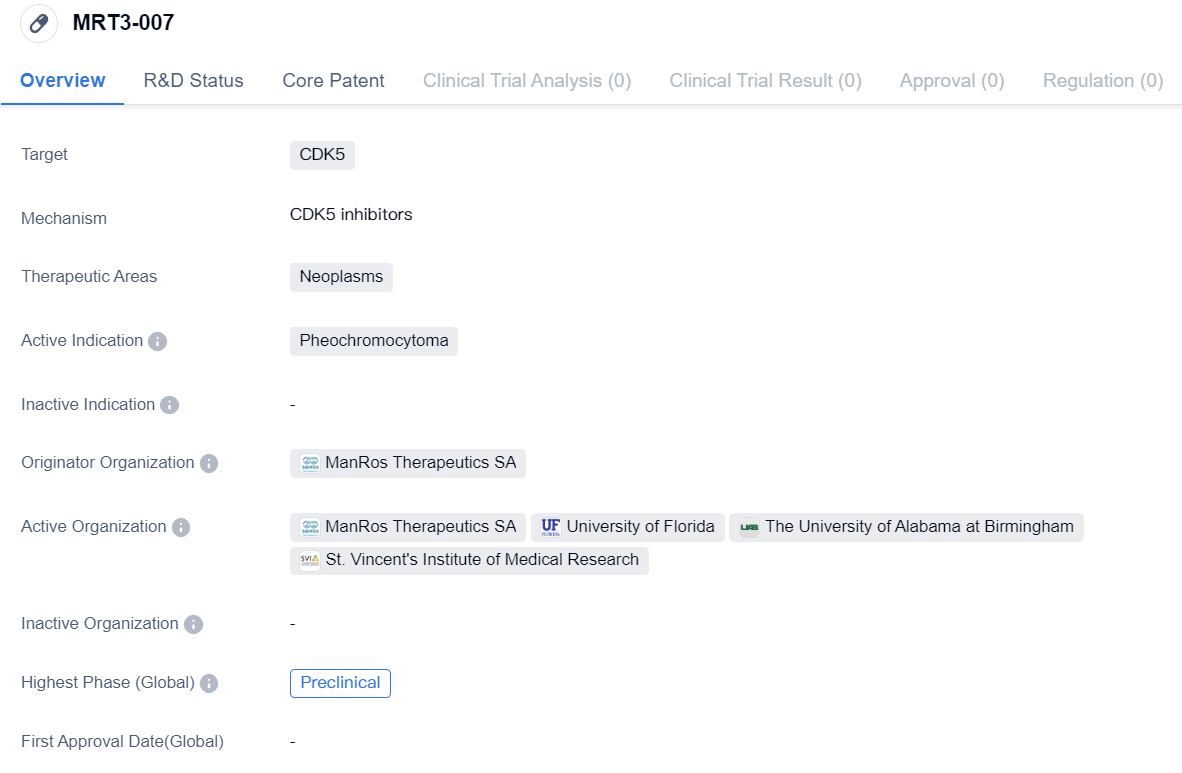
The drug MRT3-007 is a small molecule drug that targets CDK5, a protein involved in cell cycle regulation. It is being developed by ManRos Therapeutics SA, an organization specializing in biomedicine. The drug is primarily intended for the treatment of neoplasms, a broad term encompassing various types of tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Specifically, MRT3-007 is indicated for the treatment of pheochromocytoma, a rare tumor that develops in the adrenal glands. Pheochromocytomas are typically noncancerous, but they can cause symptoms such as high blood pressure, rapid heartbeat, and excessive sweating. The drug aims to address the underlying mechanisms of pheochromocytoma by targeting CDK5, which plays a role in cell proliferation and survival.
As of the latest available information, MRT3-007 is in the preclinical phase, which means it is still undergoing laboratory testing and has not yet been tested in humans. Preclinical studies are crucial for evaluating the drug's safety, efficacy, and potential side effects before proceeding to clinical trials.
The development of small molecule drugs like MRT3-007 involves the design and synthesis of chemical compounds that can interact with specific targets in the body. These drugs are typically administered orally and can penetrate cells to modulate the activity of target proteins. Small molecule drugs have been widely used in the pharmaceutical industry and have proven effective in treating various diseases.
In summary, MRT3-007 is a small molecule drug being developed by ManRos Therapeutics SA for the treatment of pheochromocytoma, a type of neoplasm. It targets CDK5, a protein involved in cell cycle regulation, and is currently in the preclinical phase of development. Further research and clinical trials will be necessary to determine the drug's safety and efficacy in humans.