CDK4 Inhibitor - A Highly Noticed Breast Cancer Treatment Drug
CDK4, or cyclin-dependent kinase 4, is a crucial protein involved in cell cycle regulation in the human body. It plays a significant role in controlling the progression of cells from the G1 phase to the S phase, where DNA replication occurs. CDK4 forms a complex with cyclin D, which activates its kinase activity. This activation leads to the phosphorylation of key proteins involved in cell cycle progression, allowing cells to transition from the resting phase to the active phase of cell division. Dysregulation of CDK4 activity has been associated with various diseases, including cancer, making it an important target for pharmaceutical interventions.
CDK4 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 76 CDK4 drugs worldwide, from 104 organizations, covering 91 indications, and conducting 1053 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.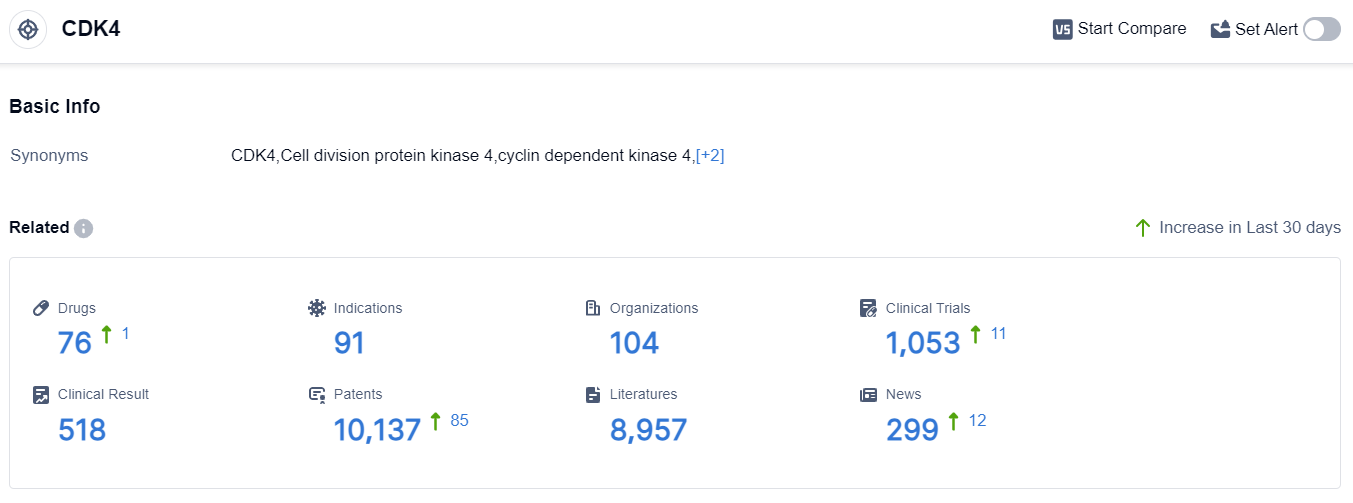
The analysis of the target CDK4 reveals a competitive landscape with multiple companies actively involved in the development of drugs. Novartis AG, Pfizer Inc., and G1 Therapeutics, Inc. are leading in terms of R&D progress.
The indications with the highest number of approved drugs include hormone receptor-positive HER2-negative breast cancer, breast cancer, and metastatic breast cancer.
Small molecule drugs are progressing rapidly under the target CDK4, indicating intense competition. Biosimilars, such as biological products, are also present, suggesting competition around the innovative small molecule drugs. The countries/locations developing fastest include China, the United States, the European Union, Australia, and Canada.
Overall, the target CDK4 presents a competitive landscape with a focus on developing drugs for various indications. Further analysis of the research and development institutions involved in biosimilar development and the progress in China will provide a comprehensive understanding of the current competitive landscape and future development opportunities for CDK4-targeted drugs.
Key Drug: Palbociclib
Palbociclib is a small molecule drug that targets CDK4 and CDK6. It has been approved for use in various therapeutic areas, including skin and musculoskeletal diseases, neoplasms, urogenital diseases, immune system diseases, digestive system disorders, endocrinology and metabolic disease, and hemic and lymphatic diseases. The drug has shown efficacy in treating metastatic breast cancer, hormone receptor positive HER2 negative breast cancer, estrogen receptor positive breast cancer, colorectal cancer, mantle-cell lymphoma, prostatic cancer, liver cancer, pancreatic cancer, renal cell carcinoma, and dermatitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
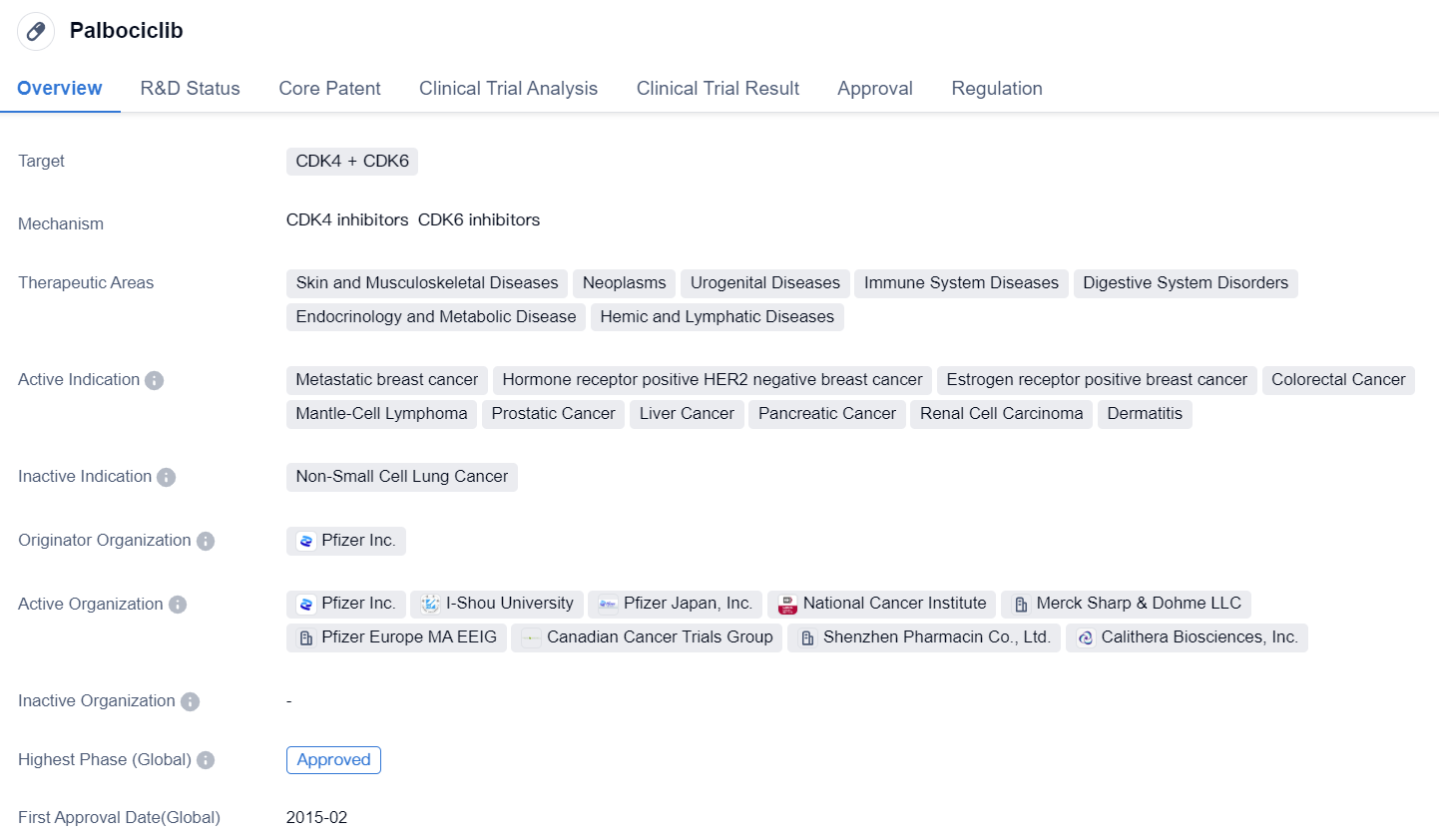 Palbociclib was developed by Pfizer Inc., a leading pharmaceutical company. It received its first approval in the United States in February 2015. The drug has also been approved in China. The regulatory process for Palbociclib involved priority review, accelerated approval, breakthrough therapy designation, and special review project.
Palbociclib was developed by Pfizer Inc., a leading pharmaceutical company. It received its first approval in the United States in February 2015. The drug has also been approved in China. The regulatory process for Palbociclib involved priority review, accelerated approval, breakthrough therapy designation, and special review project.
The approval of Palbociclib marks a significant advancement in the treatment of various cancers and other diseases. By targeting CDK4 and CDK6, the drug inhibits the progression of cancer cells and has shown promising results in clinical trials. Its approval for multiple indications demonstrates its potential to address unmet medical needs across different patient populations.
The therapeutic areas in which Palbociclib is approved cover a wide range of diseases, indicating its versatility and potential for future applications. The drug's approval for metastatic breast cancer and hormone receptor positive HER2 negative breast cancer is particularly significant, as these are common types of breast cancer that often require targeted therapies.
The originator organization, Pfizer Inc., has a strong track record in developing innovative pharmaceuticals. The approval of Palbociclib further solidifies Pfizer's position as a leader in the pharmaceutical industry.
In conclusion, Palbociclib is a small molecule drug that targets CDK4 and CDK6. It has been approved for various therapeutic areas, including multiple types of cancer and dermatitis. Developed by Pfizer Inc., the drug has received approvals in the United States and China. Its approval marks a significant advancement in the treatment of cancer and other diseases, offering new hope for patients in need.
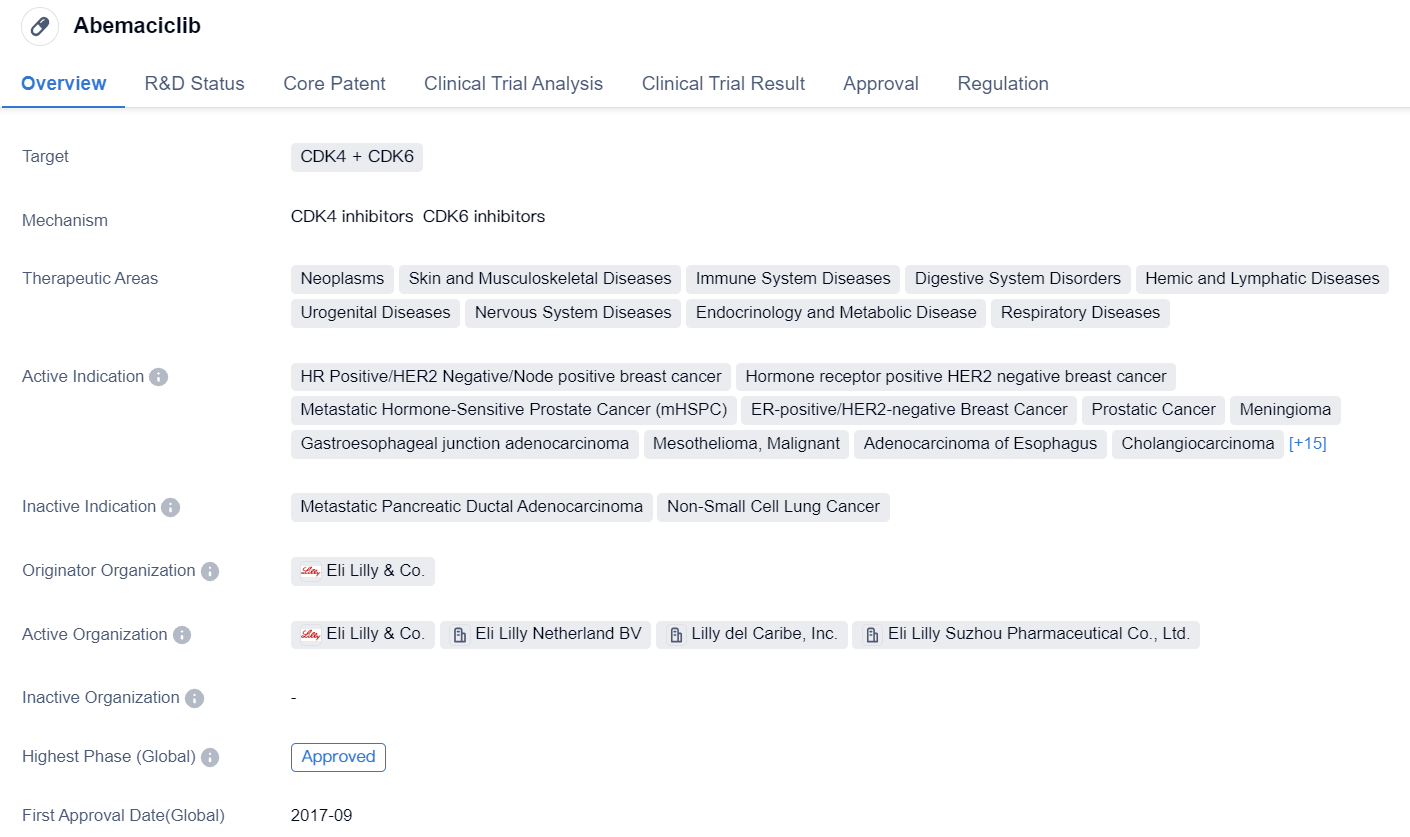
Abemaciclib
Abemaciclib is a small molecule drug that targets CDK4 and CDK6. It has been approved for various therapeutic areas including neoplasms, skin and musculoskeletal diseases, immune system diseases, digestive system disorders, hemic and lymphatic diseases, urogenital diseases, nervous system diseases, endocrinology and metabolic diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several types of cancers, such as HR positive/HER2 negative/node positive breast cancer, hormone receptor positive HER2 negative breast cancer, metastatic hormone-sensitive prostate cancer (mHSPC), ER-positive/HER2-negative breast cancer, prostatic cancer, meningioma, gastroesophageal junction adenocarcinoma, mesothelioma, malignant, adenocarcinoma of esophagus, cholangiocarcinoma, glioblastoma, endometrial carcinoma, pancreatic cancer, sarcoma, mantle-cell lymphoma, colonic cancer, retroperitoneal sarcoma, metastatic hormone refractory prostate cancer, renal cell carcinoma, colorectal cancer, melanoma, cutaneous malignant, microsatellite instability-high cancer, lymphoma, non-Hodgkin lymphoma, and PIK3CA H1047R mutant ER-positive, HER2-negative breast cancer.
Abemaciclib was developed by Eli Lilly & Co. and received its first approval in the United States in September 2017. It has also been approved in China. The drug has undergone various regulatory processes, including priority review, fast track designation, breakthrough therapy designation, and special review project.
In summary, Abemaciclib is a small molecule drug that targets CDK4 and CDK6. It has been approved for the treatment of multiple cancers and has shown promising results in various therapeutic areas. Its approval in both the United States and China highlights its global significance. The drug has undergone rigorous regulatory processes, indicating its potential as a breakthrough therapy.