Advances in Clinical Research on Amylase Inhibitor
Amylase is an essential enzyme found in the human body that plays a crucial role in the digestion of carbohydrates. Produced primarily in the salivary glands and pancreas, amylase breaks down complex starch molecules into simpler sugars like glucose, maltose, and dextrin. This process begins in the mouth, where salivary amylase initiates the breakdown of starches into smaller fragments. Once in the stomach, the enzyme continues its action until it reaches the small intestine, where pancreatic amylase takes over to complete the digestion process. Without amylase, the body would struggle to efficiently convert carbohydrates into usable energy, leading to potential digestive issues and nutrient deficiencies.
Amylase Competitive Landscape
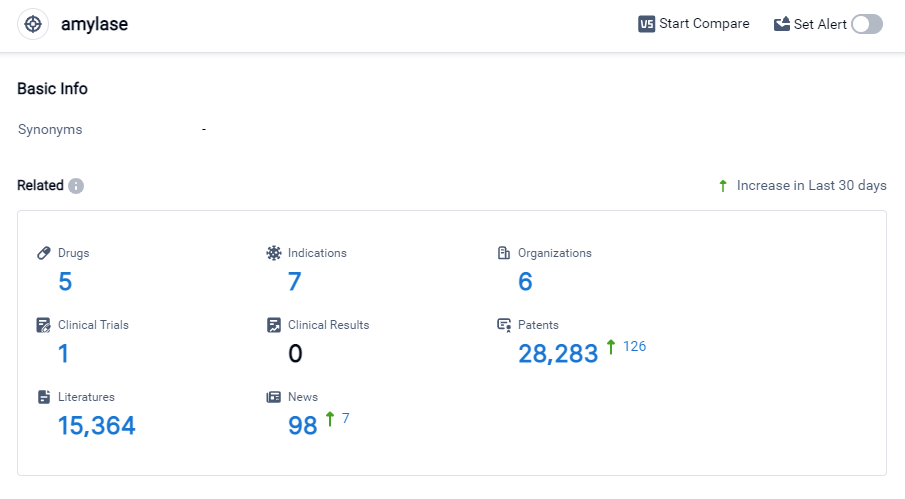
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 5 amylase drugs worldwide, from 6 organizations, covering 7 indications, and conducting 1 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the provided data, the current competitive landscape of target amylase indicates that companies like Anagram Therapeutics, Inc. and Propanc Biopharma, Inc. are growing rapidly in their R&D efforts. Drugs targeting amylase have been approved for indications such as Exocrine Pancreatic Insufficiency, Neoplasms, Ovarian Cancer, Solid Tumors, Pancreatic Cancer, Malabsorption Syndromes, and Gastrointestinal Diseases. Enzyme-based drugs and small molecule drugs are progressing rapidly, indicating potential competition around innovative drugs. The countries/locations with significant development in target amylase include the United States, European Union, Australia, Spain, France, Denmark, and Belgium. However, the progress in China cannot be determined based on the provided data. Overall, the target amylase market shows promising growth potential with active R&D efforts and a diverse range of indications and drug types.
Key drug: Acarbose
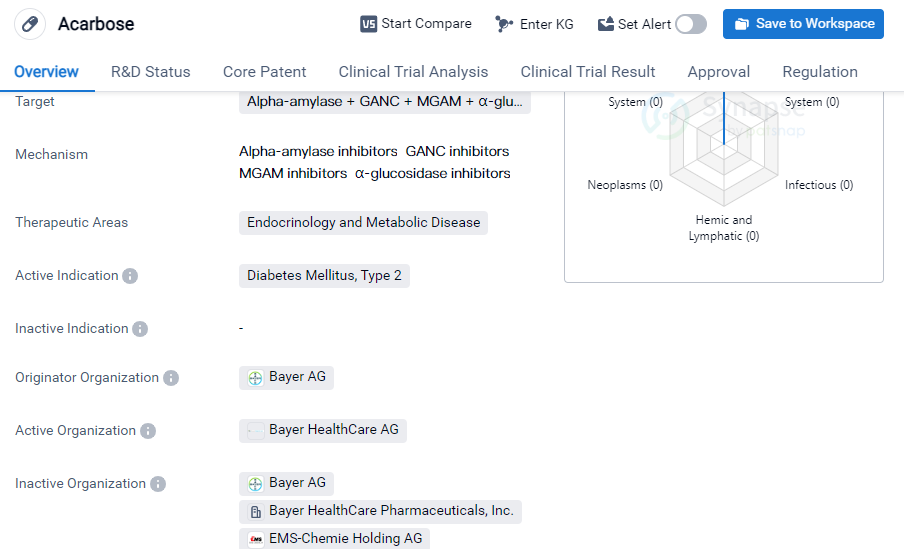
Acarbose is a small molecule drug that falls under the therapeutic areas of endocrinology and metabolic disease. It is primarily used for the treatment of diabetes mellitus, specifically type 2 diabetes. The drug targets several enzymes including alpha-amylase, GANC, MGAM, and α-glucosidase.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Acarbose is Bayer AG, a well-known pharmaceutical company. The drug has reached the highest phase of approval globally, indicating its successful development and regulatory progress. Acarbose received its first approval in the United States in September 1995, making it available to patients in that country.
One notable aspect of Acarbose is its regulation status, which is classified as priority review. This suggests that the drug has undergone an expedited evaluation process by regulatory authorities, possibly due to its potential benefits and urgency in addressing the medical needs of patients with type 2 diabetes.
Acarbose's mechanism of action involves inhibiting the enzymes targeted, which play a role in the digestion and absorption of carbohydrates. By inhibiting these enzymes, Acarbose helps to slow down the breakdown of complex carbohydrates into simple sugars, thereby reducing the postprandial rise in blood glucose levels. This mechanism makes Acarbose a valuable treatment option for individuals with type 2 diabetes, as it helps to control their blood sugar levels.
In summary, Acarbose is a small molecule drug developed by Bayer AG for the treatment of type 2 diabetes. It targets multiple enzymes involved in carbohydrate digestion and absorption, and its regulatory status as a priority review drug highlights its potential significance in managing this metabolic disease. Acarbose received its first approval in the United States in 1995 and has since been approved in other countries, including China. Its successful development and regulatory progress make it a valuable addition to the pharmaceutical industry's arsenal in combating diabetes mellitus.