Advances in Clinical Research on Cholinesterase Inhibitors
Cholinesterase is a class of glycoprotein that is present in the body in various isoenzyme forms. It can be generally divided into true cholinesterase and pseudocholinesterase. True cholinesterase, also known as acetylcholinesterase, mainly exists in the cholinergic nerve synaptic cleft, especially in the folds of the post-synaptic membrane of the motor nerve end plate, but also in cholinergic neurons and red blood cells. This enzyme has the highest activity and specificity for physiological concentrations of Acetylcholine (Ach). One enzyme molecule can hydrolyze 3 x 10 molecules of Ach, and it is often simply referred to as cholinesterase. Pseudocholinesterase is widely found in neuroglial cells, plasma, liver, kidney, and intestines. It has lower specificity for Ach and can hydrolyze other cholinergic esters, such as succinylcholine.
The active center on the surface of the cholinesterase protein molecule has two binding sites for acetylcholine, which are the negatively charged anionic site and the esterase site. The esterase site contains an acidic site composed of serine hydroxyl groups and a basic site composed of a histidine imidazole ring, which are bound by hydrogen bonds, enhancing the nucleophilic activity of the serine hydroxyl groups, making them easier to bind with acetylcholine. The process of cholinesterase hydrolysis of acetylcholine can be divided into three steps: ① the quaternary ammonium ion head with a positive charge in the acetylcholine molecule structure is bound to the anionic site of the cholinesterase by electrostatic attraction. Simultaneously, the carbonyl carbon in the acetylcholine molecule is covalently bound to the hydroxyl group of the serine of the cholinesterase esterase site, forming a complex of acetylcholine and cholinesterase. ② The complex of acetylcholine and cholinesterase then splits into choline and acetylated cholinesterase. ③ Acetylated cholinesterase is rapidly hydrolyzed, releasing acetic acid, and restoring the enzyme’s activity.
Cholinesterase Competitive Landscape
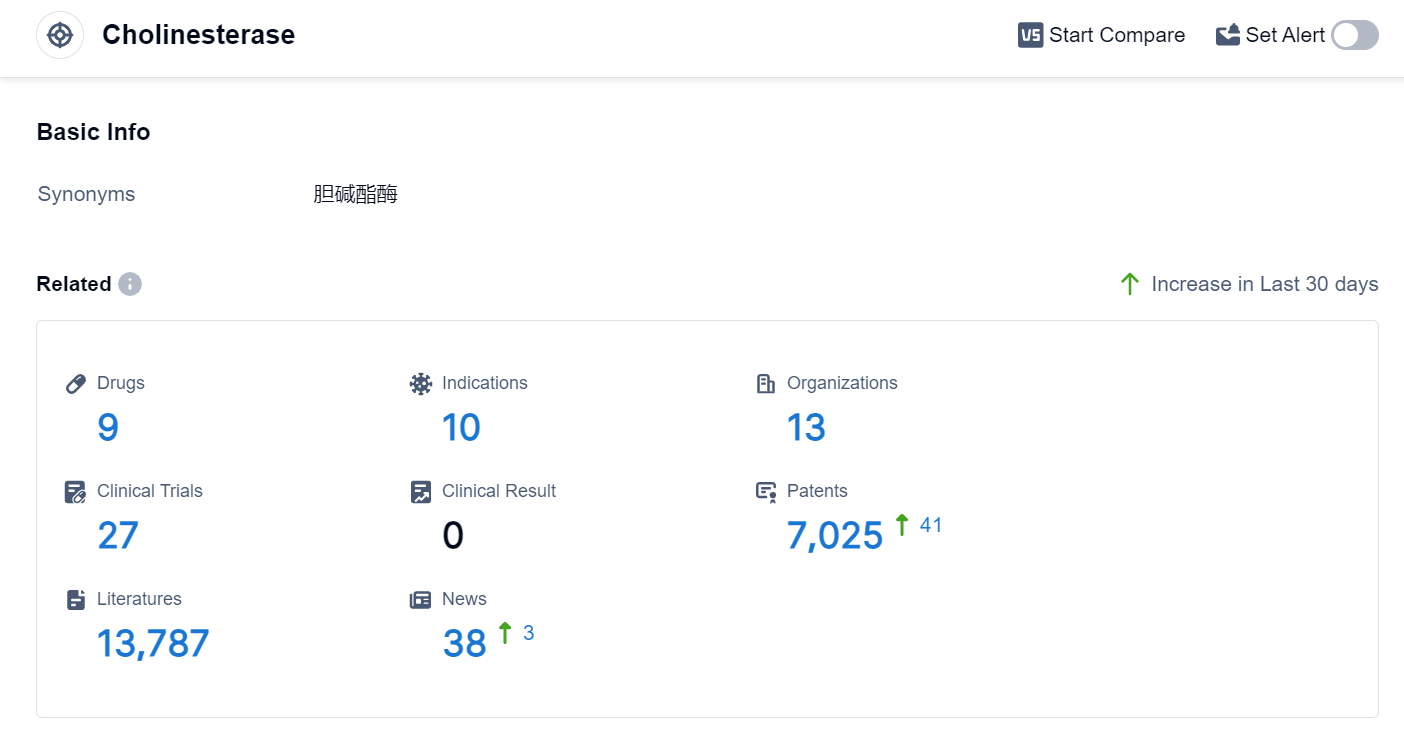
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 24 Sep 2023, there are a total of 9 Cholinesterase drugs worldwide, from 13 organizations, covering 10 indications, and conducting 27 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target Cholinesterase reveals that Alpha Cognition, Inc., Hyundai Pharmaceutical Co., Ltd., and Galantos Pharma GmbH are the companies growing fastest under this target. The highest stage of development is Phase 3, with drugs targeting Alzheimer's Disease. Small molecule drugs are progressing rapidly, indicating a focus on this drug type. The countries/locations developing fastest include Japan, China, South Korea, and Canada. China has shown significant progress in the development of drugs targeting Cholinesterase. Overall, the competitive landscape for the target Cholinesterase is dynamic, with ongoing R&D efforts and a focus on Alzheimer's Disease and other relevant indications.
The Globally Approved Cholinesterase Inhibitor: Pyrantel Pamoate
Pyrantel Pamoate is a small molecule drug that falls under the therapeutic area of infectious diseases. It targets cholinesterase, an enzyme involved in the breakdown of acetylcholine, a neurotransmitter. The drug is primarily indicated for the treatment of several parasitic infections, including Ancylostomiasis, Trichostrongylosis, Ascariasis, and Enterobiasis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
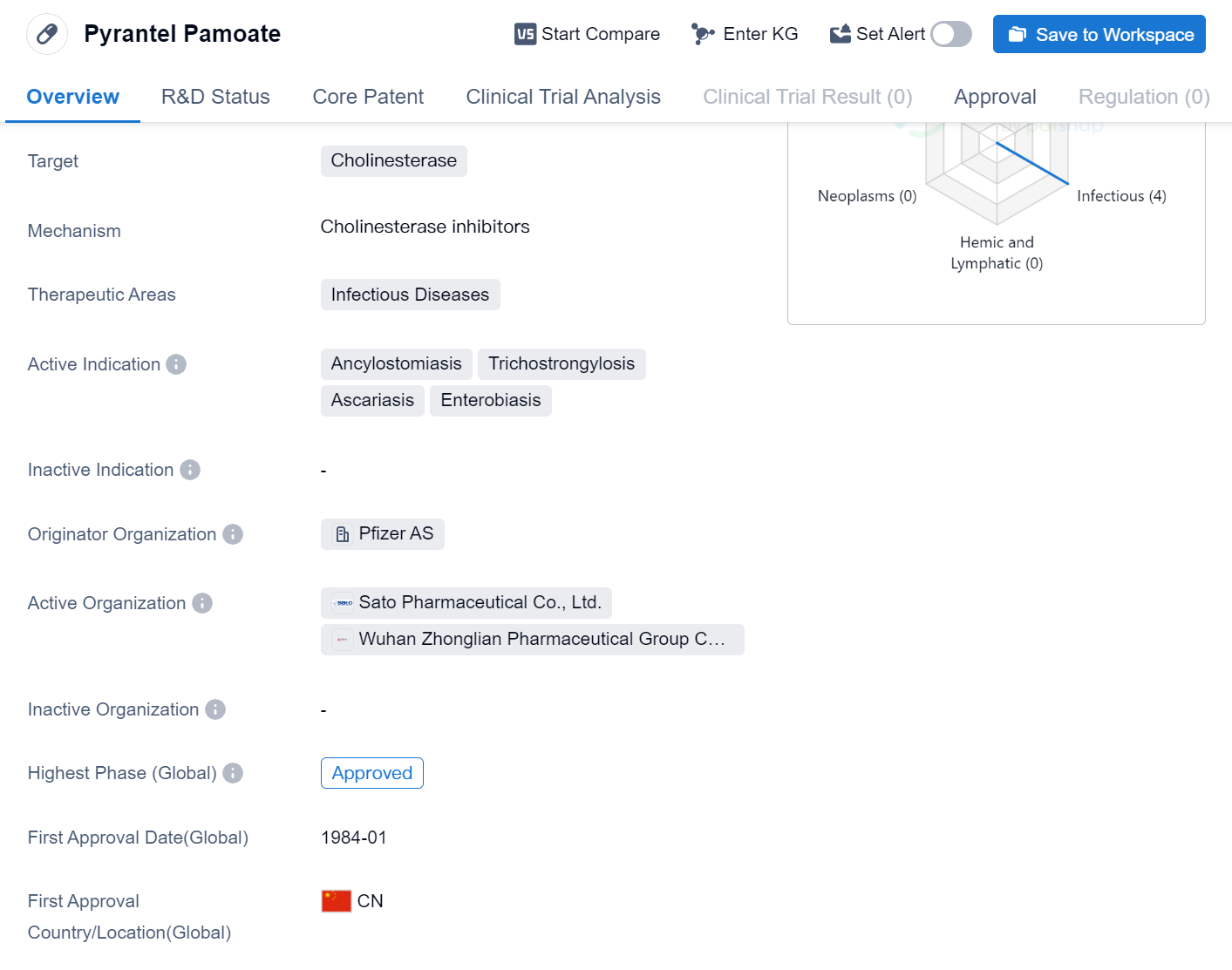
Pyrantel Pamoate was first approved in China in January 1984, making it one of the earliest drugs in its class to receive regulatory approval. The drug is manufactured by Pfizer AS, a well-known pharmaceutical organization with a strong presence in the global market. It is important to note that Pyrantel Pamoate has achieved the highest phase of approval globally.
Ancylostomiasis, also known as hookworm infection, is caused by parasitic worms that attach themselves to the intestinal wall and feed on blood. Trichostrongylosis is another parasitic infection caused by nematodes that primarily affect livestock but can also infect humans. Ascariasis is caused by the roundworm Ascaris lumbricoides and is one of the most common parasitic infections worldwide. Enterobiasis, commonly known as pinworm infection, is caused by the parasitic worm Enterobius vermicularis and is highly prevalent, especially in children.
The approval of Pyrantel Pamoate in China suggests its effectiveness in treating these parasitic infections prevalent in the region. The drug's mechanism of action, targeting cholinesterase, disrupts the nervous system of the parasites, leading to their paralysis and subsequent elimination from the body.
Given the drug's long history of approval and its indication for multiple parasitic infections, Pyrantel Pamoate has likely established itself as a reliable treatment option in the field of infectious diseases. Its approval in the global market further highlights its potential to address the significant burden of parasitic infections in various populations.
In conclusion, Pyrantel Pamoate is a small molecule drug developed by Pfizer AS, targeting cholinesterase and primarily indicated for the treatment of Ancylostomiasis, Trichostrongylosis, Ascariasis, and Enterobiasis. With its earliest approval in China in 1984, the drug has achieved the highest phase of approval globally. Its approval suggests its potential as an effective treatment option for parasitic infections prevalent in the region.