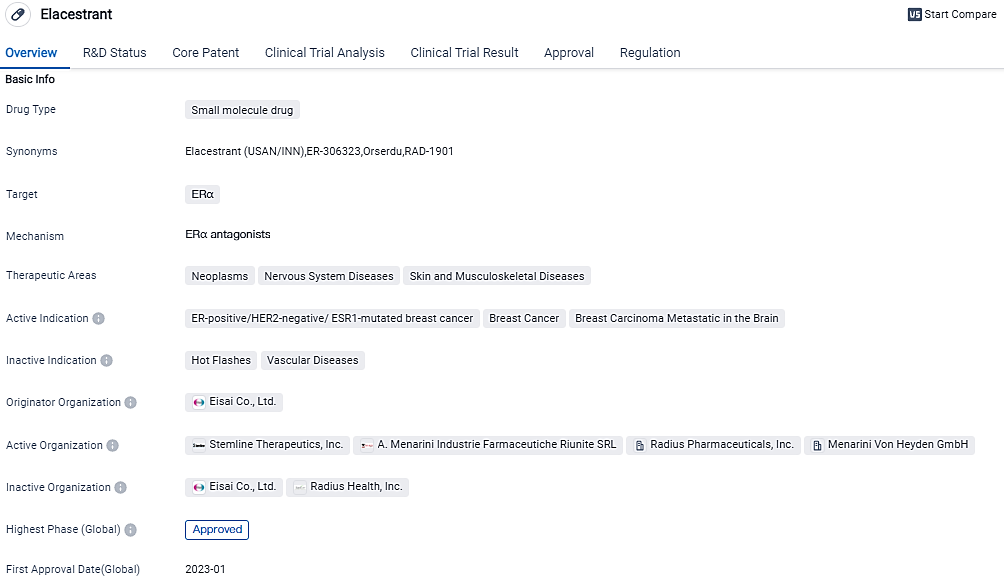
The Menarini Group's ORSERDU® (Elacestrant) has received approval from the EC for breast cancer
The Menarini Group, a global pharmaceutical and diagnostics corporation, together with their fully owned subsidiary Stemline Therapeutics Inc., declared that ORSERDU® (elacestrant) has received authorisation from the European Commission. This monotherapy is designated for postmenopausal women as well as men who are diagnosed with estrogen receptor (ER)–positive, HER2-negative, locally advanced or metastatic breast cancer with a stimulating ESR1 mutation. This approval comes post the patients showing disease advancement despite receiving at least one line of endocrine therapy that includes a CDK 4/6 inhibitor.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The European Commission's approval comes on the heels of the Medicinal Products for Human Use within the EMA's positive feedback given in July 2023. This validation positions ORSERDU as the premier and unique therapy explicitly recommended for managing ER+, HER2- tumors embodying ESR1 mutations.
ESR1 mutations occur due to the exposure to endocrine therapy, appearing in nearly 40% of ER+, HER2- mBC inflicted patients. These are a popular catalyst for resistance to the standard endocrine treatment, turning the tumors concealing these mutations more challenging to address until now.
"Patients suffering from metastatic breast cancer have long sought substantial, bearable alternatives that would treat their ailment while allowing them to dedicate their energies to things holding importance for them," asserted Elcin Barker Ergun, the Chief Executive Officer of the Menarini Group. "Our achievement lies in offering a cutting-edge breast cancer treatment that delivers effectiveness through a daily pill, signaling the first breakthrough in endocrine therapy in close to twenty years."
"This approval paves the way for us to have an unparalleled treatment alternative that directly counters the mutations that render this kind of breast cancer more challenging to manage, serving as a beacon of hope for our patients and their kin," expressed Giuseppe Curigliano, MD, PhD, a Medical Oncology Professor at the University of Milano, and the Director of the Early Drug Development division at the European Institute of Oncology, IRCCS, Italy.
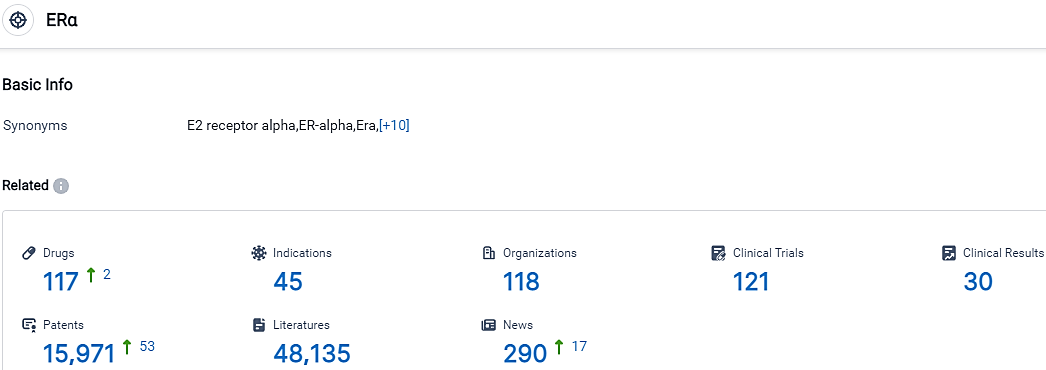
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 117 investigational drugs for the ERα target, including 45 indications,118 R&D institutions involved, with related clinical trials reaching 121,and as many as 15871 patents.
ORSERDU (elacestrant) with estrogen receptor (ER)-positive, HER2-negative, locally advanced or metastatic breast cancer with an activating ESR1 mutation who have disease progression following at least one line of endocrine therapy including a CDK 4/6 inhibitor. The Menarini Group obtained global licensing rights for elacestrant in July 2020 from Radius Health, Inc. The Menarini Group is now fully responsible for global registration, commercialization, and further development activities for elacestrant.