Advances in Clinical Research on Leukotriene Receptor Antagonist
CysLT, or cysteinyl leukotrienes, are inflammatory mediators produced by various cells in the human body, including mast cells, eosinophils, and macrophages. These molecules play a crucial role in the pathophysiology of asthma and allergic diseases. CysLTs bind to specific receptors, such as CysLT1 and CysLT2, leading to bronchoconstriction, increased mucus production, and recruitment of inflammatory cells. This results in airway inflammation, narrowing of the airways, and subsequent respiratory symptoms. Understanding the role of CysLT in the human body has paved the way for the development of targeted therapies, such as leukotriene receptor antagonists, which effectively alleviate symptoms and improve the quality of life for individuals with asthma and allergic conditions.
Leukotriene receptor antagonists are an effective class of asthma treatment drugs. They can alleviate asthma symptoms, improve lung function, and reduce asthma exacerbation. They are effective in treating mild to moderate asthma, exercise-induced asthma, and cold-air-induced asthma, especially suitable for the treatment of patients with aspirin-sensitive asthma. Leukotriene receptor antagonists can be used as a long-term medication for patients with mild, moderate, and severe asthma, but their effect as single-drug treatment for asthma is not as good as glucocorticoids and long-acting inhaled β2 receptor agonists. However, they can reduce the dose of inhaled glucocorticoids consumed by patients with moderate to severe asthma, thereby improving the clinical efficacy of inhaled glucocorticoids and reducing side effects caused by large doses of hormones. Currently, leukotriene receptor antagonists have been used for the treatment of patients with asthma combined with allergic rhinitis, and the efficacy is good.
Leukotriene Receptor Competitive Landscape
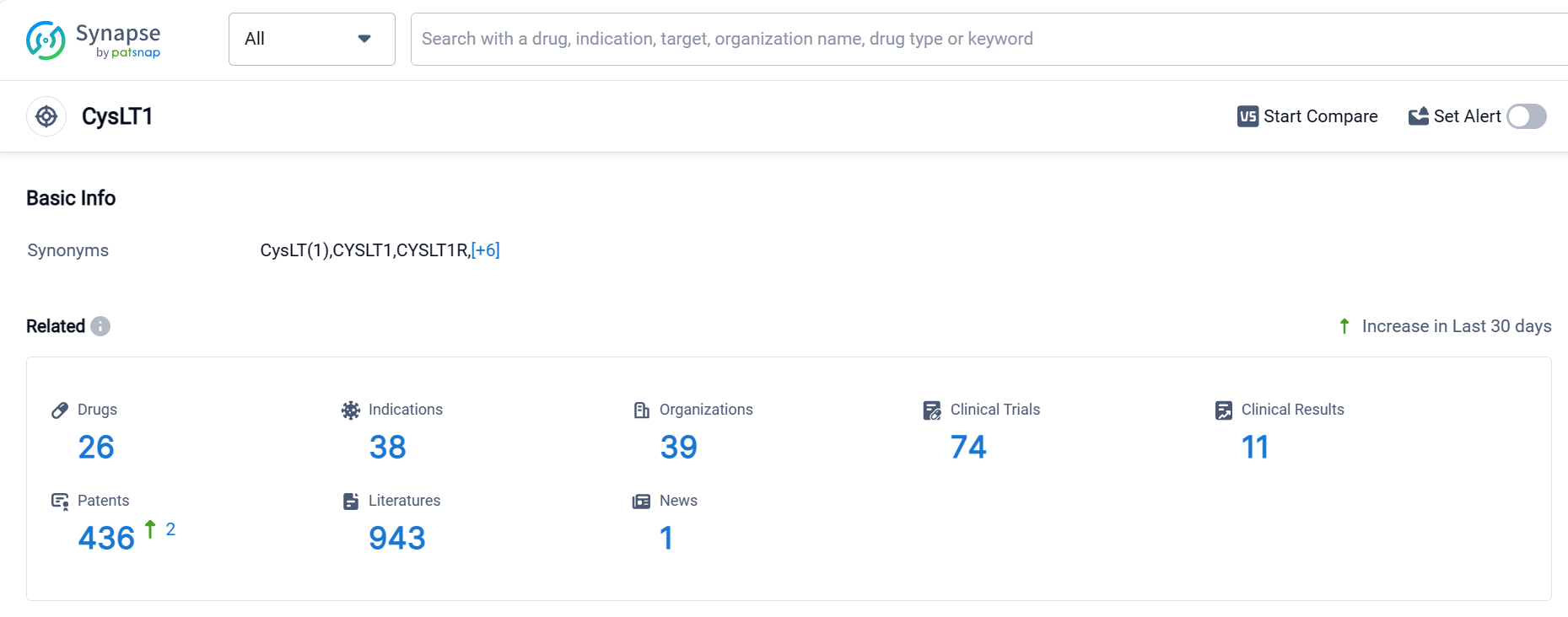
According to Patsnap Synapse, as of 1 Oct 2023, there are a total of 45 CysLT drugs worldwide, from 41 organizations, covering 21 indications, and conducting 540 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of target CysLT reveals that Merck & Co., Inc., Organon & Co., and KYORIN Pharmaceutical Co., Ltd. are leading in terms of R&D progress and approved drugs. Asthma is the most common approved indication for drugs targeting CysLT. Small molecule drugs are progressing rapidly, indicating their effectiveness in targeting CysLT. The United States, Japan, and China are the countries with significant progress in the development of drugs targeting CysLT. Overall, the competitive landscape for target CysLT is dynamic, with potential for future development and innovation in the pharmaceutical industry.
Key drug: Pranlukast Hydrate
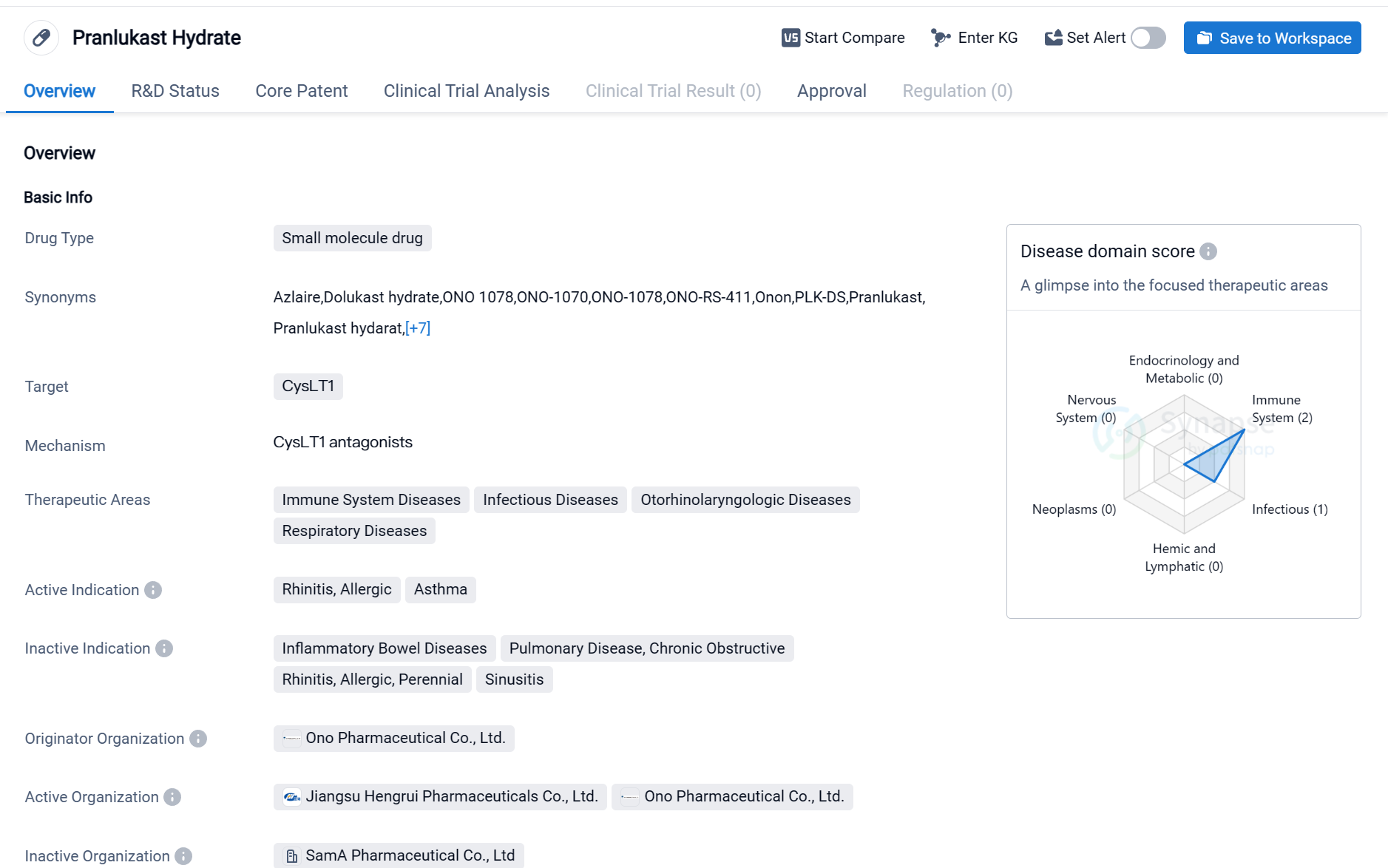
Pranlukast Hydrate is a small molecule drug that targets CysLT1, a receptor involved in immune system diseases, infectious diseases, otorhinolaryngologic diseases, and respiratory diseases. It is primarily used to treat rhinitis, allergic reactions, and asthma. The drug was developed by Ono Pharmaceutical Co., Ltd., a pharmaceutical company based in Japan.
Pranlukast Hydrate received its first approval in Japan in March 1995, making it available for use in the country. It has also obtained approvals in other countries, indicating its global recognition and acceptance. The drug has reached the highest phase of development, which is the approved stage globally.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Pranlukast Hydrate is designed to interact with specific molecular targets in the body, in this case, the CysLT1 receptor. By targeting this receptor, the drug aims to modulate the immune response and alleviate symptoms associated with immune system diseases, infectious diseases, otorhinolaryngologic diseases, and respiratory diseases.
The therapeutic areas of Pranlukast Hydrate highlight its potential to address a range of conditions related to the immune system, infections, and respiratory health. Rhinitis, allergic reactions, and asthma are the active indications for which the drug has been approved. These conditions often involve inflammation and immune system dysregulation, and Pranlukast Hydrate may help manage symptoms and improve patient outcomes.
Ono Pharmaceutical Co., Ltd. is the originator organization behind Pranlukast Hydrate. As the developer of the drug, the company has played a crucial role in its research, development, and commercialization. Ono Pharmaceutical Co., Ltd. is a reputable pharmaceutical company known for its contributions to the field of biomedicine.
In summary, Pranlukast Hydrate is a small molecule drug developed by Ono Pharmaceutical Co., Ltd. It targets the CysLT1 receptor and is approved for the treatment of rhinitis, allergic reactions, and asthma. The drug has obtained approvals in multiple countries, including Japan, where it was first approved in 1995. Its therapeutic areas include immune system diseases, infectious diseases, otorhinolaryngologic diseases, and respiratory diseases. Pranlukast Hydrate represents a significant advancement in the pharmaceutical industry, offering potential benefits for patients suffering from these conditions.