Mabwell Discloses Initial Patient Treatment in Phase Ib/II Study for Nectin-4 Focused ADC coupled with PD-1 Inhibitor
Mabwell, a pioneer in the biopharmaceutical sector with a comprehensive industry chain, declared the administration of the first patient dosage in a Phase Ib/II study. This trial involves their novel Nectin-4 targeting ADC (9MW2821), used together with a PD-1 inhibitor to manage locally advanced or metastatic urothelial carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This study is designed to assess the safety, tolerability, initial efficacy, and pharmacokinetic characteristics of 9MW2821 when used with the PD-1 inhibitor in patients struggling with advanced local or metastatic urothelial carcinoma.
9MW2821 is a novel Nectin-4 targeting ADC developed by world-class ADC development platform and automated high-throughput antibody discovery platform of Mabwell. It achieves site-determined modifications of the antibody using trademarked conjugate technology linkers and an enhanced ADC conjugation method. 9MW2821 can attach specifically to Nectin-4 on the cellular membrane surface, internalize and spring a cytotoxic drug, leading to the programmed death of cancer cells.
9MW2821 brings the benefits of a uniform composition, greater purity, and is adaptable for industrial enlargement. Initial results point towards solid tumours yielding positive therapeutic results and having a sound safety profile at the recommended phase II dosage.
Mabwell is currently encouraging enrollment from multiple cohorts for urothelial carcinoma, cholangiocarcinoma, prostate cancer, HER-2 negative breast cancer, and non-small cell lung cancer. The R&D developments of 9MW2821 are leading in China and are ranked second globally. 9MW2821 leads in garnering initial clinical data in cervical cancer among similar targeted treatments internationally.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
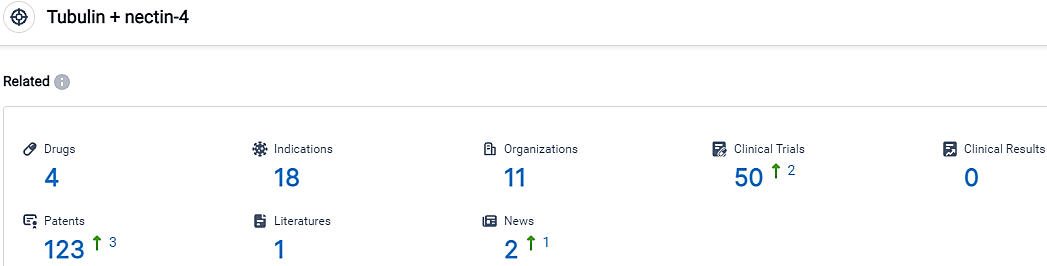
According to the data provided by the Synapse Database, As of October 8, 2023, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 18 indications,11 R&D institutions involved, with related clinical trials reaching 50,and as many as 123 patents.
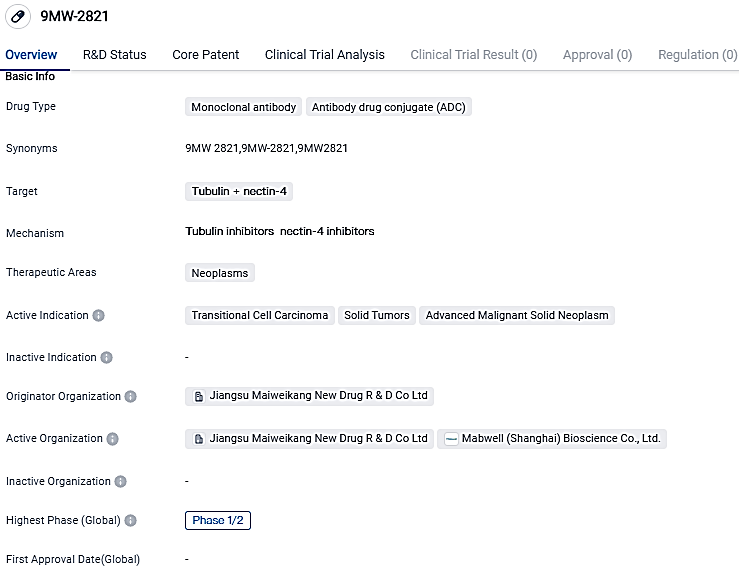
9MW2821 is a Monoclonal antibody and Antibody drug conjugate that targets Tubulin and nectin-4. The drug shows potential in treating neoplasms, including Transitional Cell Carcinoma, Solid Tumors, and Advanced Malignant Solid Neoplasm. Further research and clinical trials will be necessary to determine its safety and efficacy in treating these conditions.