Advances in Clinical Research on Nrf2 Inhibitor
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a type of basic region-leucine zipper (bZIP) transcription factors, encoded by the NFE2L2 gene. Nrf2 can form a heterodimer with small musculoaponeurotic fibrosarcoma (sMAF) protein, which can recognize and bind to the antioxidant response element (ARE) located in the regulatory region to enhance the transcription of target genes.
Generally, genes regulated by Nrf2 mainly participate in oxidative stress regulation, including the glutathione system, the thioredoxin antioxidant system, and enzymes related to phase I, II, and III drug metabolism. By inducing the transcription of these genes, Nrf2 can activate the cellular defense system and protect cells from exogenous and endogenous attacks.
Therefore, Nrf2 is considered a core transcription factor regulating cell protection systems. In addition to playing a critical role in cell protection, Nrf2 also regulates the transcription of various functional genes, especially those related to cellular metabolism. Nrf2 regulates the metabolism of lipids, carbohydrates, nucleotides, and amino acids by controlling the expression of key enzymes.
Moreover, it participates in the regulation of several proteasomal subunit and autophagy gene expression, further enhancing Nrf2's impact on the metabolic regulation network. In general, the activation of Nrf2 can enhance synthetic metabolic pathways, which is beneficial for cancer cell proliferation. During tumor growth and development, Nrf2 activation can increase tumor cells' antioxidant and drug metabolism capabilities, thus improving tumor survival rates. Besides, Nrf2 participates in tumor metabolic network changes, making tumors more inclined to undergo aerobic glycolysis. Nrf2 is overactivated in various different types of tumor cells.
Nrf2, or nuclear factor erythroid 2-related factor 2, is a transcription factor that plays a crucial role in the human body's defense against oxidative stress and inflammation. It regulates the expression of various antioxidant and detoxification genes, promoting cellular protection and maintaining redox homeostasis. Nrf2 activation is essential for combating harmful free radicals and reactive oxygen species, which can lead to various diseases, including cancer, neurodegenerative disorders, and cardiovascular conditions. Understanding and harnessing the potential of Nrf2 signaling pathways have become a significant focus in pharmaceutical research, aiming to develop novel therapeutic interventions for oxidative stress-related diseases.
Nrf2 Competitive Landscape
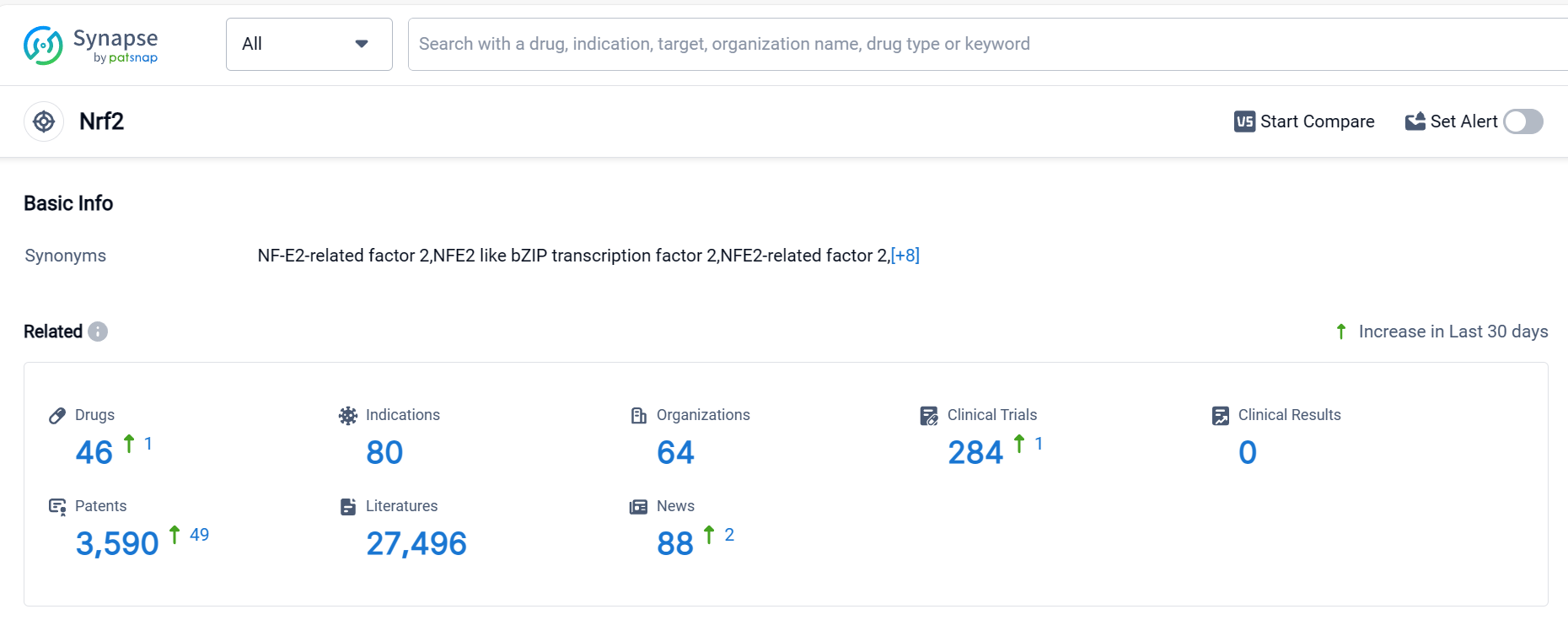
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 46 Nrf2 drugs worldwide, from 64 organizations, covering 80 indications, and conducting 284 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target Nrf2 in the pharmaceutical industry reveals that companies like Biogen, Inc., Reata Pharmaceuticals, Inc., and Teva Pharmaceutical Industries Ltd. are actively involved in the development of drugs targeting Nrf2. Multiple indications have been approved for drugs under the target Nrf2, including Multiple Sclerosis, Friedreich Ataxia, Plaque psoriasis, and more. Small molecule drugs are progressing rapidly, and the presence of biosimilars is not mentioned in the provided data. The United States, European Union, and Japan are leading in the development of drugs targeting Nrf2, with China also making progress. Overall, the competitive landscape for target Nrf2 is dynamic, with potential for future development and innovation in the pharmaceutical industry.
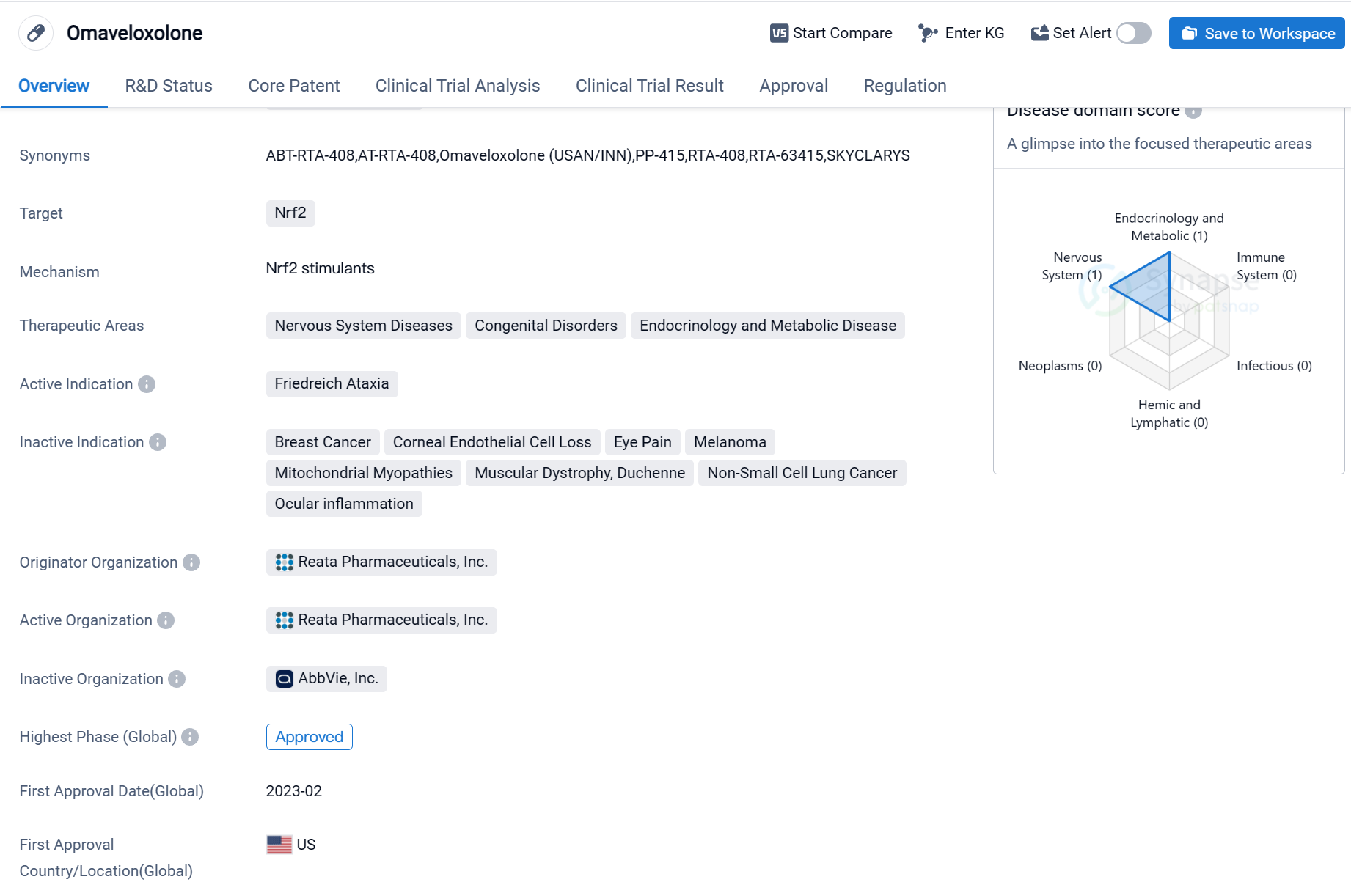
Key drug:Omaveloxolone
Omaveloxolone is a small molecule drug that targets Nrf2, a protein involved in cellular defense mechanisms. It is primarily used in the treatment of Friedreich Ataxia, a rare genetic disorder that affects the nervous system. The drug is developed by Reata Pharmaceuticals, Inc., a pharmaceutical company specializing in the development of novel therapeutics.
Omaveloxolone has received approval for use in the United States, with the first approval date set for February 2023. The drug has been granted several regulatory designations, including Rare Pediatric Disease, Fast Track, and Orphan Drug status. These designations aim to facilitate the development and expedite the review process for drugs that address unmet medical needs in rare diseases.
The therapeutic areas targeted by Omaveloxolone extend beyond Friedreich Ataxia and include Nervous System Diseases, Congenital Disorders, and Endocrinology and Metabolic Disease. This suggests that the drug may have potential applications in treating a range of conditions within these therapeutic areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Omaveloxolone is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting. This characteristic often allows for efficient manufacturing processes and potentially lower production costs compared to larger biologic drugs.
The approval of Omaveloxolone represents a significant milestone for Reata Pharmaceuticals, Inc. and highlights their commitment to developing innovative therapies for rare diseases. The drug's regulatory designations further emphasize the urgent need for effective treatments in the field of rare pediatric diseases.
In summary, Omaveloxolone is a small molecule drug developed by Reata Pharmaceuticals, Inc. that targets Nrf2. It has received approval for the treatment of Friedreich Ataxia in the United States and holds regulatory designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug. The drug shows promise in addressing various therapeutic areas, including Nervous System Diseases, Congenital Disorders, and Endocrinology and Metabolic Disease. Its approval marks a significant achievement for Reata Pharmaceuticals, Inc. and underscores the importance of developing treatments for rare diseases.