The latest data on Sunvozertinib, an EGFR inhibitor from Dizal Pharmaceutical, made its debut at the 2023 ESMO Conference
On October 23, 2023, Dizal Pharmaceutical announced that the latest data from the pooled analysis of efficacy and safety of the company’s first proprietary new targeted drug for lung cancer, an EGFR inhibitor named Sunvozertinib for first-line treatment of EGFR exon20ins mutation type advanced NSCLC, debuted at the 2023 European Society for Medical Oncology (ESMO) meeting. The pooled analysis research includes first-line treatment patients of EGFR exon20ins mutation type advanced NSCLC from the global multicenter phase I/II trials WU-KONG1 and the phase II trial WU-KONG15 initiated by Chinese researchers.
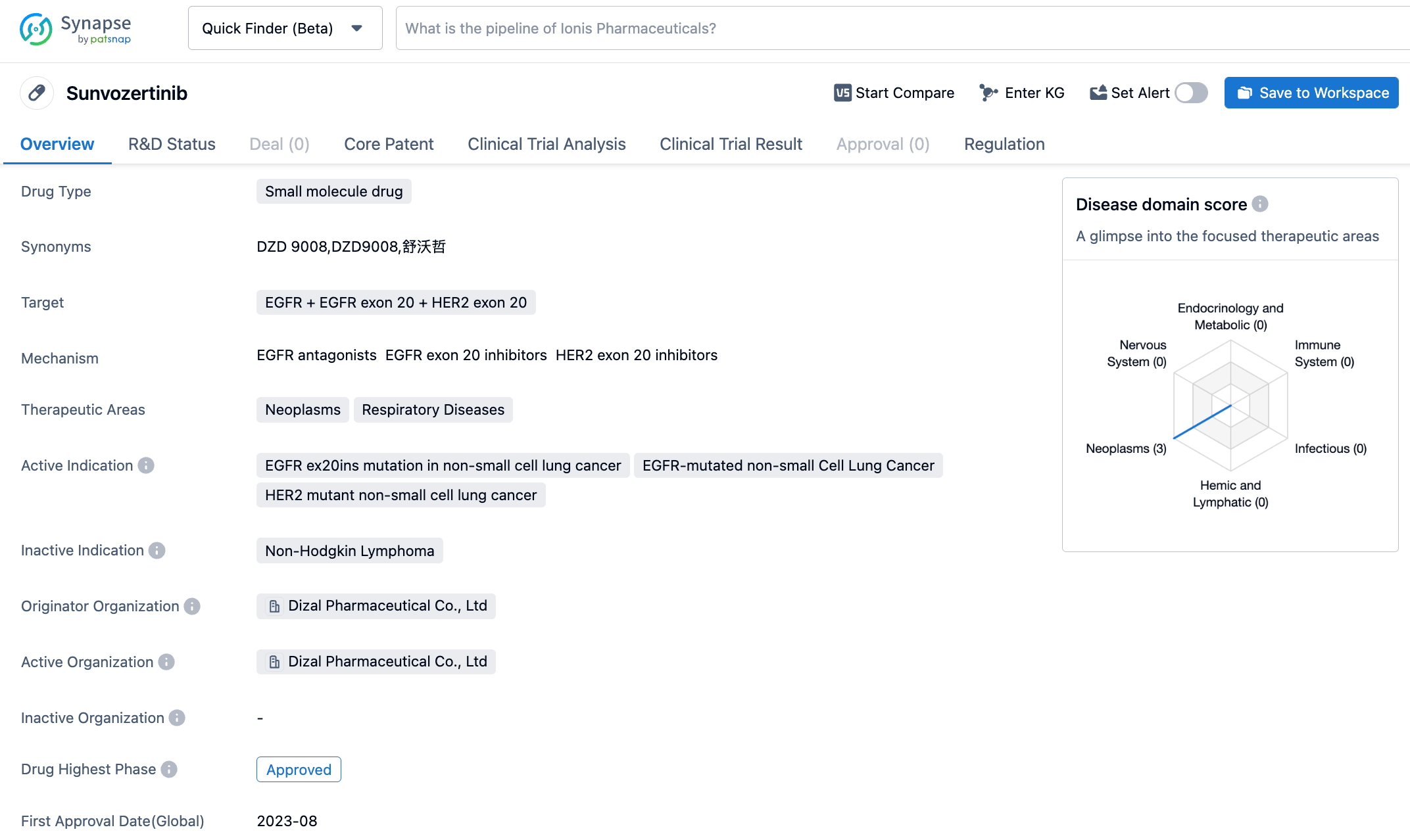
Sunvozertinib is a highly selective EGFR tyrosine kinase inhibitor (TKI) targeting multiple EGFR mutation subtypes developed by Dizal Pharmaceuticals. It is also a Class 1 new drug for advanced NSCLC with EGFR exon20ins mutation. Sunvozertinib, having 5-aminopyrimidine as the parent ring and a more flexible phenylamine structure at the C-4 position, results in high affinity and selectivity for EGFR exon 20 insertion mutations. Previously, based on its excellent efficacy and safety, Sunvozertinib® became the first and currently the only Class 1 new drug originating from China to receive "breakthrough therapy designation" in both China and the United States in the field of lung cancer, and its marketing application was included in NMPA's priority review.
Researchers' evaluation results showed that among the 28 patients included in the efficacy analysis, 100% observed a reduction in the size of the target lesion. The confirmed objective response rate (ORR) for first-line monotherapy with Sunvozertinib® for late-stage NSCLC with EGFR exon20ins mutation was as high as 78.6%. Among them, the median progression-free survival (mPFS) in the 300 mg group was 12.4 months, breaking previous research reports. Sunvozertinib® monotherapy demonstrated potent and durable antitumor activity, and showed good antitumor activity in patients with various EGFR exon20ins mutation subtypes, highlighting the best potential in its class. Additionally, Sunvozertinib® was well-tolerated, the overall safety was consistent with previous second-line or later reports, and was similar to traditional EGFR-TKIs.
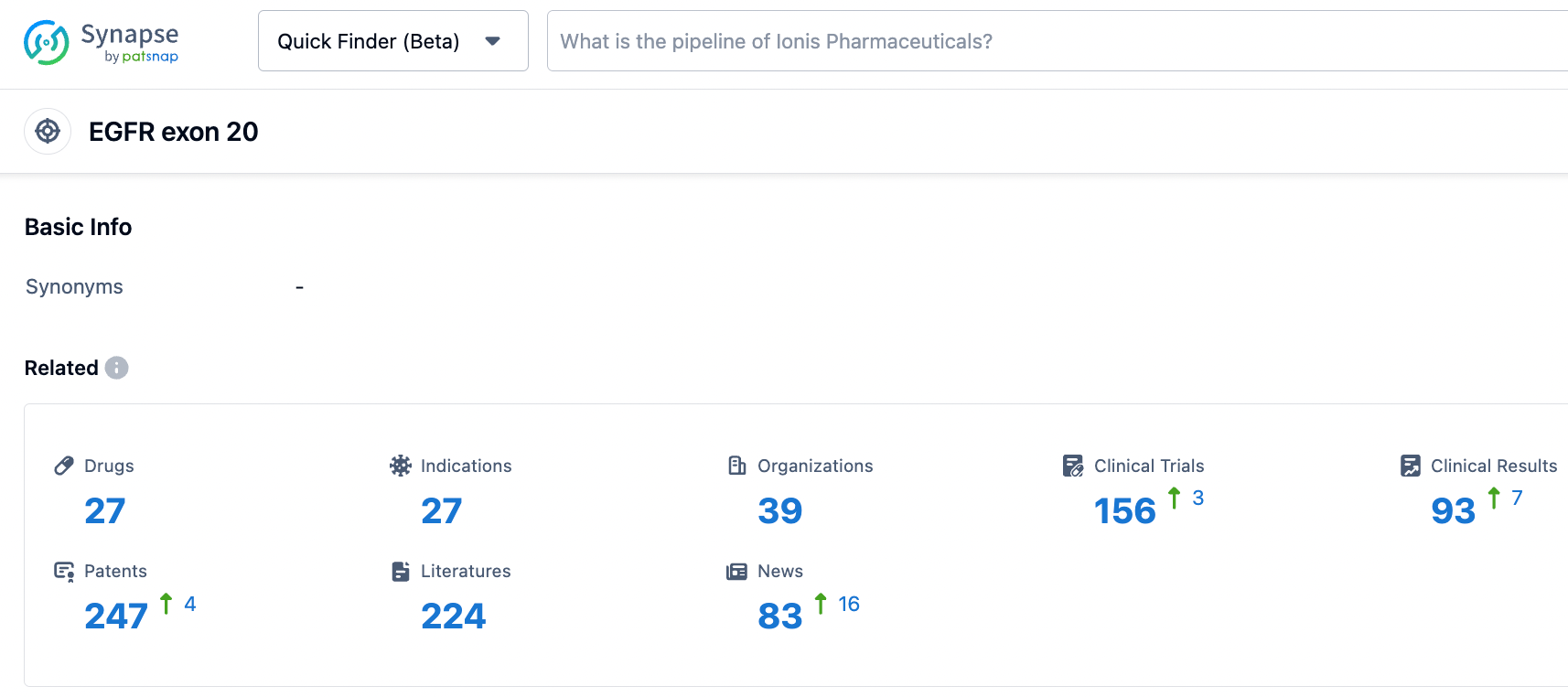
According to the information disclosed by the synapse database, as of October 24, 2023, there are 27 investigational drugs for the EGFR exon 20 target, with 27 indications, 39 research institutes involved, 156 related clinical trials, and as many as 247 patents... Besides Sunvozertinib, Osimertinib, Amivantamab, and Fomiventanib, as three third-generation EGFR-TKIs, have been approved for first-line treatment of patients with EGFR mutated NSCLC. In addition, in May 2023, Bédafortinib's second-line indication was approved, competing with Sunvozertinib for the third-generation EGFR-TKI pattern.