Advances in Clinical Research on PrEP drug
Pre-exposure prophylaxis (PrEP) is a preventive measure that involves the use of antiretroviral drugs to reduce the risk of HIV infection in individuals who are not infected but are at high risk of exposure. After taking PrEP medication, the active ingredients enter the cells and interrupt the reverse transcription process during HIV invasion, inhibiting the synthesis of HIV viral DNA from viral RNA, and preventing HIV from entering the cell nucleus, thus avoiding HIV infection. Continuous use of PrEP medication prevents the virus from progressing to the established stage of infection and spreading in the body. It is important to note that PrEP is not a vaccine, and the body does not produce HIV antibodies as a result of using PrEP. Therefore, regular oral administration is necessary to achieve its preventive effect. Taking a pill before engaging in sexual activity does not effectively block HIV transmission.
Can PrEP effectively prevent HIV?
When taken regularly and consistently, PrEP medication can effectively prevent HIV. According to the 2011 iPrEx clinical trial conducted among gay and bisexual men in the United States, PrEP demonstrated a preventive efficacy of 96%-99% in individuals who were highly adherent to the medication regimen. However, if the medication is not taken consistently or in sufficient doses, its effectiveness diminishes. Research has also shown that for heterosexual individuals with an HIV-positive partner, PrEP can reduce the risk of infection by up to 75%. It is important to note that the effectiveness of PrEP depends on the adherence of the individual taking it. Strict adherence to the medication regimen is necessary to minimize the risk of HIV infection.
It is worth noting that PrEP provides biomedical protection against HIV and has limitations in preventing drug-resistant strains of the virus. Therefore, the use of PrEP medication should be accompanied by the consistent use of condoms to reduce the risk of HIV and other sexually transmitted infections. Additionally, individuals taking PrEP medication should undergo sexual health and HIV testing every three months, and their medication adherence and high-risk behaviors should be closely monitored in the long term.
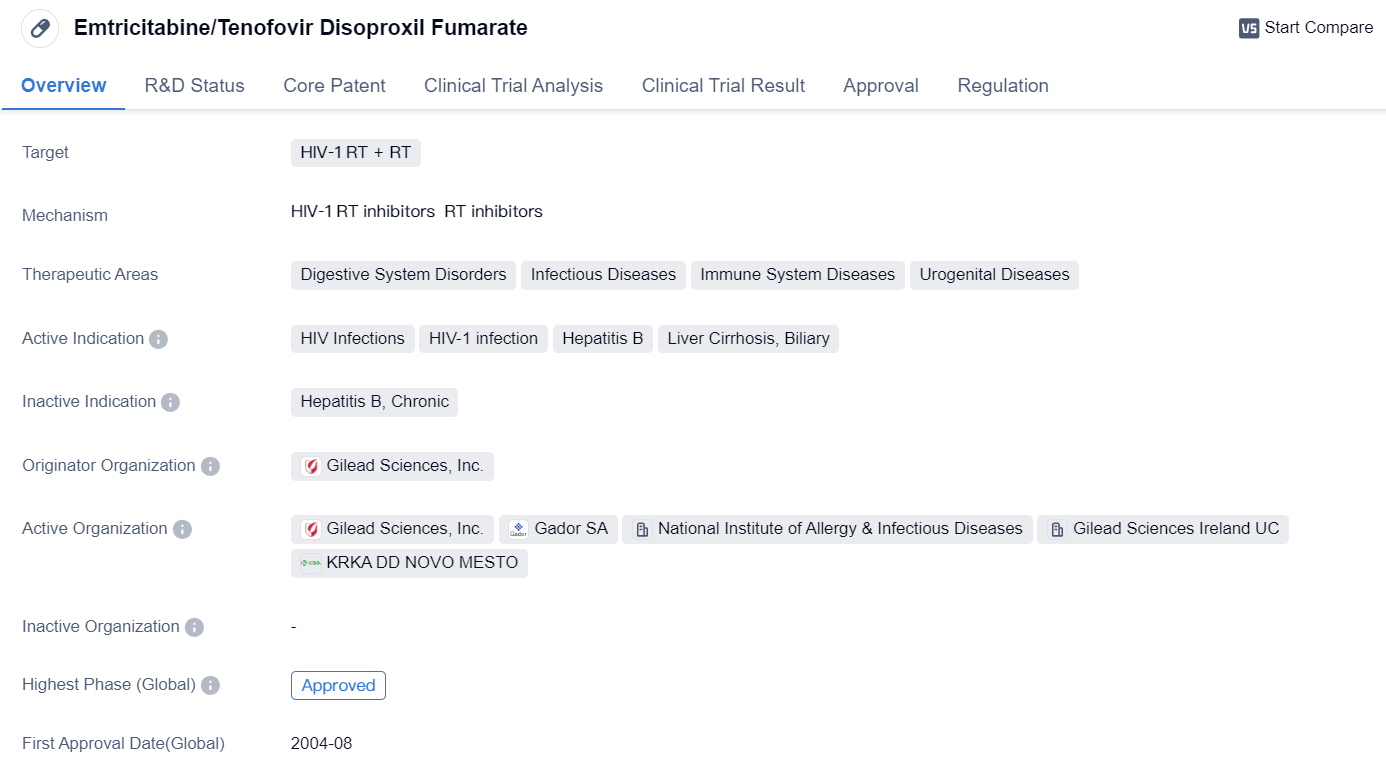
Key PrEP Drug: Emtricitabine/Tenofovir Disoproxil Fumarate
Emtricitabine/Tenofovir Disoproxil Fumarate is a small molecule drug used in the treatment of various conditions related to HIV-1 infection, hepatitis B, liver cirrhosis, and biliary disorders. It is primarily developed by Gilead Sciences, Inc. and has received approvals in both the global and Chinese markets.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This drug targets HIV-1 reverse transcriptase (RT) and has shown efficacy in inhibiting the replication of the virus. It is indicated for the treatment of HIV infections, including HIV-1 infection, and has been approved for use in the United States since August 2004. The drug's mechanism of action involves blocking the reverse transcriptase enzyme, which is essential for the replication of the HIV virus.
Emtricitabine/Tenofovir Disoproxil Fumarate falls under the therapeutic areas of digestive system disorders, infectious diseases, immune system diseases, and urogenital diseases. These areas encompass a wide range of conditions related to the gastrointestinal tract, infections, immune system dysfunctions, and disorders of the urinary and reproductive systems.
In terms of regulatory status, the drug has received accelerated approval and fast track designation. Accelerated approval is a regulatory pathway that allows for the expedited approval of drugs that address unmet medical needs, while fast track designation is a program designed to facilitate the development and expedite the review of drugs for serious conditions.
The approval of Emtricitabine/Tenofovir Disoproxil Fumarate in both the global and Chinese markets highlights its significance in the treatment of HIV-1 infection and related conditions. The drug's approval in the United States in 2004 indicates its early recognition as an effective treatment option for HIV infections.
In summary, Emtricitabine/Tenofovir Disoproxil Fumarate is a small molecule drug developed by Gilead Sciences, Inc. It targets HIV-1 reverse transcriptase and is indicated for the treatment of HIV infections, hepatitis B, liver cirrhosis, and biliary disorders. The drug has received approvals in both the global and Chinese markets, with its first approval in the United States in 2004. Its regulatory status includes accelerated approval and fast track designation, reflecting its importance in addressing unmet medical needs and expediting the drug development process.
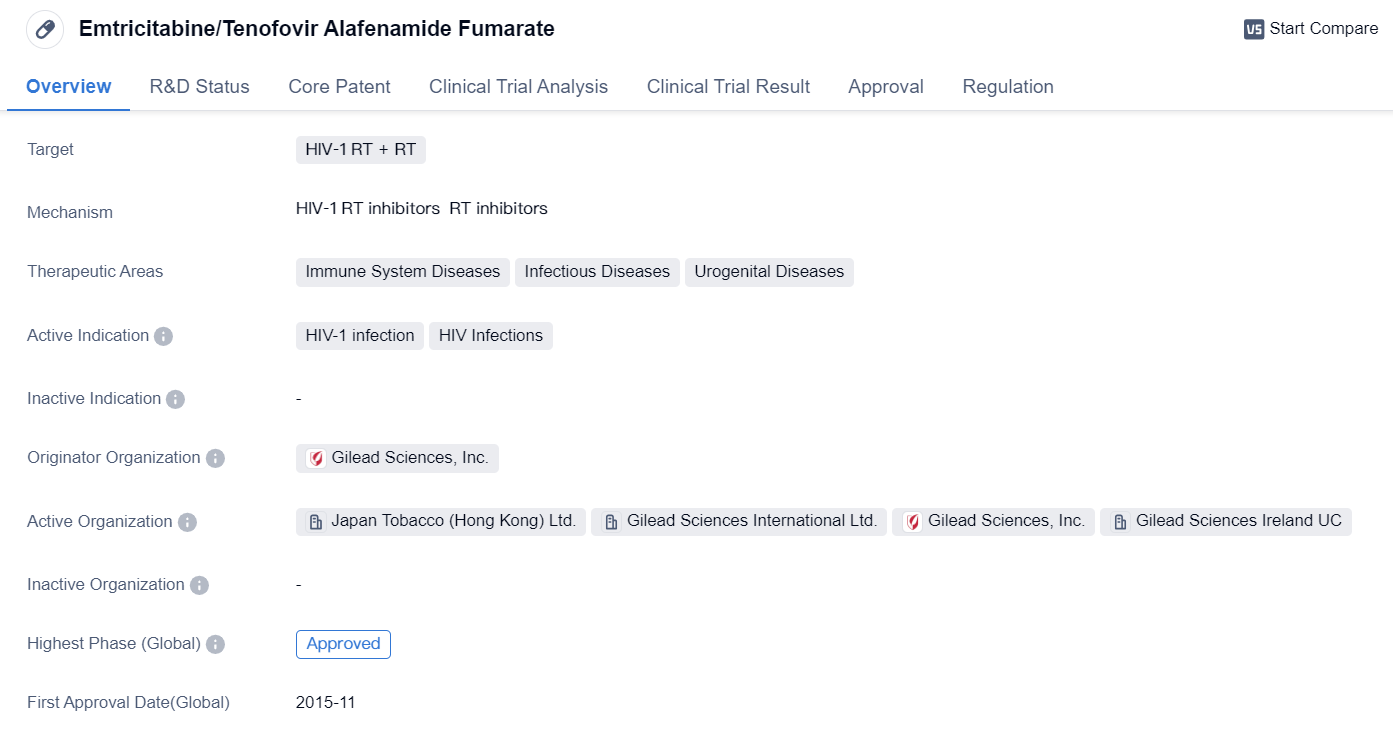
Emtricitabine/Tenofovir Alafenamide Fumarate
Emtricitabine/Tenofovir Alafenamide Fumarate is a small molecule drug used in the treatment of HIV-1 infection. It targets the HIV-1 reverse transcriptase enzyme, which plays a crucial role in the replication of the virus. The drug is designed to inhibit the activity of this enzyme, thereby reducing the viral load and slowing down the progression of the disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas of Emtricitabine/Tenofovir Alafenamide Fumarate include immune system diseases, infectious diseases, and urogenital diseases. These areas are directly related to the impact of HIV-1 infection on the human body. By targeting the virus and reducing its replication, the drug helps to manage the immune system, prevent the spread of the infection, and alleviate the symptoms associated with the disease.
Gilead Sciences, Inc. is the originator organization of Emtricitabine/Tenofovir Alafenamide Fumarate. The drug has received approval for use in multiple countries, including Canada, where it was first approved in November 2015. It is important to note that the drug has also received orphan drug designation, indicating its potential to treat rare diseases or conditions.
As a small molecule drug, Emtricitabine/Tenofovir Alafenamide Fumarate is likely to have a well-defined chemical structure and a relatively small molecular weight. This characteristic allows for easier absorption, distribution, and metabolism within the body, enhancing its therapeutic efficacy.
The approval of Emtricitabine/Tenofovir Alafenamide Fumarate in both global and Chinese markets highlights its effectiveness and safety profile. The drug has undergone rigorous clinical trials and regulatory processes to ensure its quality, efficacy, and safety for patients.
In summary, Emtricitabine/Tenofovir Alafenamide Fumarate is a small molecule drug developed by Gilead Sciences, Inc. for the treatment of HIV-1 infection. It targets the HIV-1 reverse transcriptase enzyme and has been approved for use in multiple countries, including Canada. With its therapeutic focus on immune system diseases, infectious diseases, and urogenital diseases, the drug offers a promising treatment option for individuals living with HIV-1 infection.