Adverum Biotechnologies reported encouraging Aflibercept Protein level results from their LUNA Phase 2 study
Adverum Biotechnologies, Inc., a firm operating in the clinical phase exploring the establishment of gene therapy as a fresh, routine treatment for widespread eye disorders, has released early findings on aflibercept protein activity from its ongoing Phase 2 LUNA study. This research is currently assessing ixoberogene soroparvovec's efficacy in treating wet age-related macular degeneration.
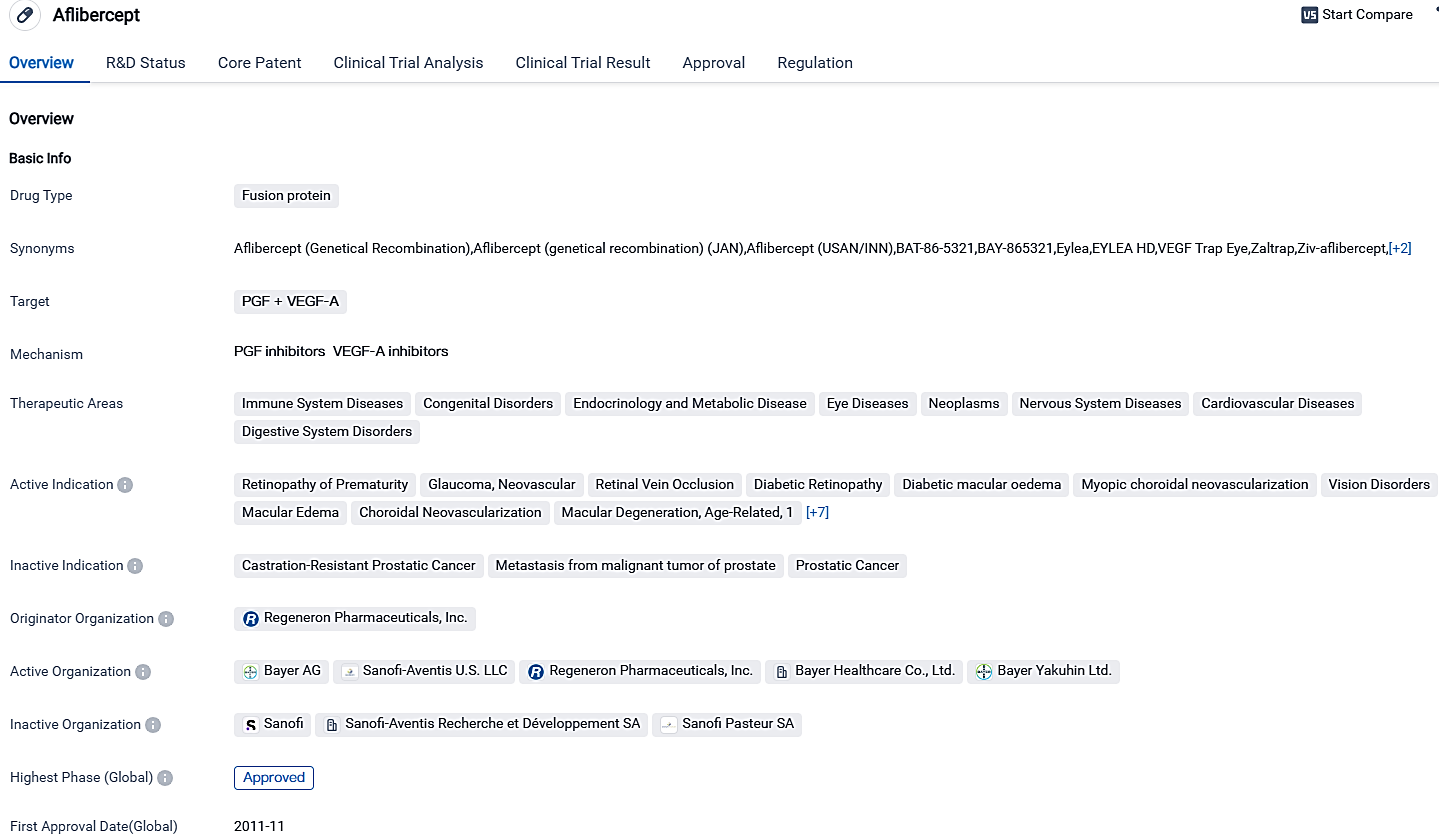
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The newly released findings today reveal aflibercept protein amounts for both the 2E11 and 6E10 vg/eye dosages, suggesting that both dosages fall within the effective therapeutic range. Importantly, these protein expression quantities align with those seen in the OPTIC trial, where sustained aflibercept levels and corresponding clinical manifestations have been seen in patients for several years post single injection with Ixo-vec.
"This initial LUNA trial data is heartening, demonstrating comparable aflibercept expression amounts at the 2E11 and 6E10 dosages," affirmed Laurent Fischer, M.D., president and chief executive officer of Adverum Biotechnologies. “Contrary to the current care standard, which demands multiple bolus injections to sustain adequate amounts of aflibercept, gene therapy could significantly minimize treatment load by maintaining lasting therapeutic levels of anti-VEGF."
Dr. Fischer went on to say, "We are enthusiastic about the optimistic emerging clinical activity and safety outcomes from the LUNA trial. At this year's AAO, we anticipate sharing updated enduring follow-up data from the OPTIC trial, revealing protein quantities of aflibercept spanning four years. Additionally, we hope to offer preliminary LUNA clinical activity and safety findings after a considerable patient subset has finished the corticosteroid prophylactic taper regimens."
“Ixo-vec employs the 7m8 capsid, crafted through directed evolution to boost retinal transduction, and delivers therapy where it is most required. The findings from the LUNA study have the potential to be clinically significant, as the measured aflibercept amounts in this trial are on par with the enduring aflibercept amounts experienced by patients in the OPTIC trial.” stated Arshad M. Khanani, M.D., M.A., managing partner and clinical research director, Sierra Eye Associates.
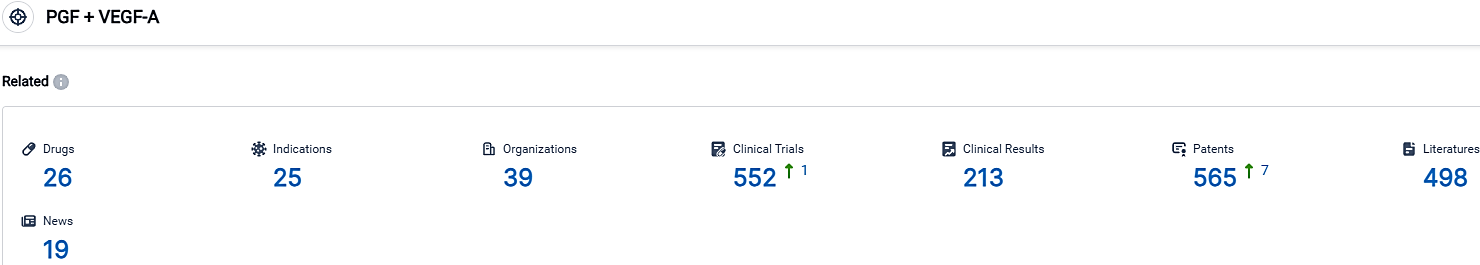
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 3, 2023, there are 26 investigational drugs for the PGF and VEGF-A target, including 25 indications,39 R&D institutions involved, with related clinical trials reaching 552,and as many as 565 patents.
Aflibercept is a medical substance that zeroes in on both PGF and VEGF-A. Its acknowledged use in numerous remedial regions and indications demonstrates potential in managing a diverse scope of diseases and conditions. The pharmaceutical has procured authorization owing to its perceived advantages, with its acceptance in multiple nations indicating its international influence in biomedicine.