AEON Biopharma Reveals Strategic Shift to Focus on ABP-450 Biosimilar Development
AEON Biopharma, Inc., a company in the clinical-stage of biopharmaceutical development, is dedicated to advancing a botulinum toxin complex targeting several therapeutic conditions. AEON Biopharma revealed their intention to proceed with a crucial clinical development trial in cervical dystonia for their main product, ABP-450 (prabotulinumtoxinA) injection. They will be using the 351 regulatory pathway for biosimilars, with BOTOX being the reference product.
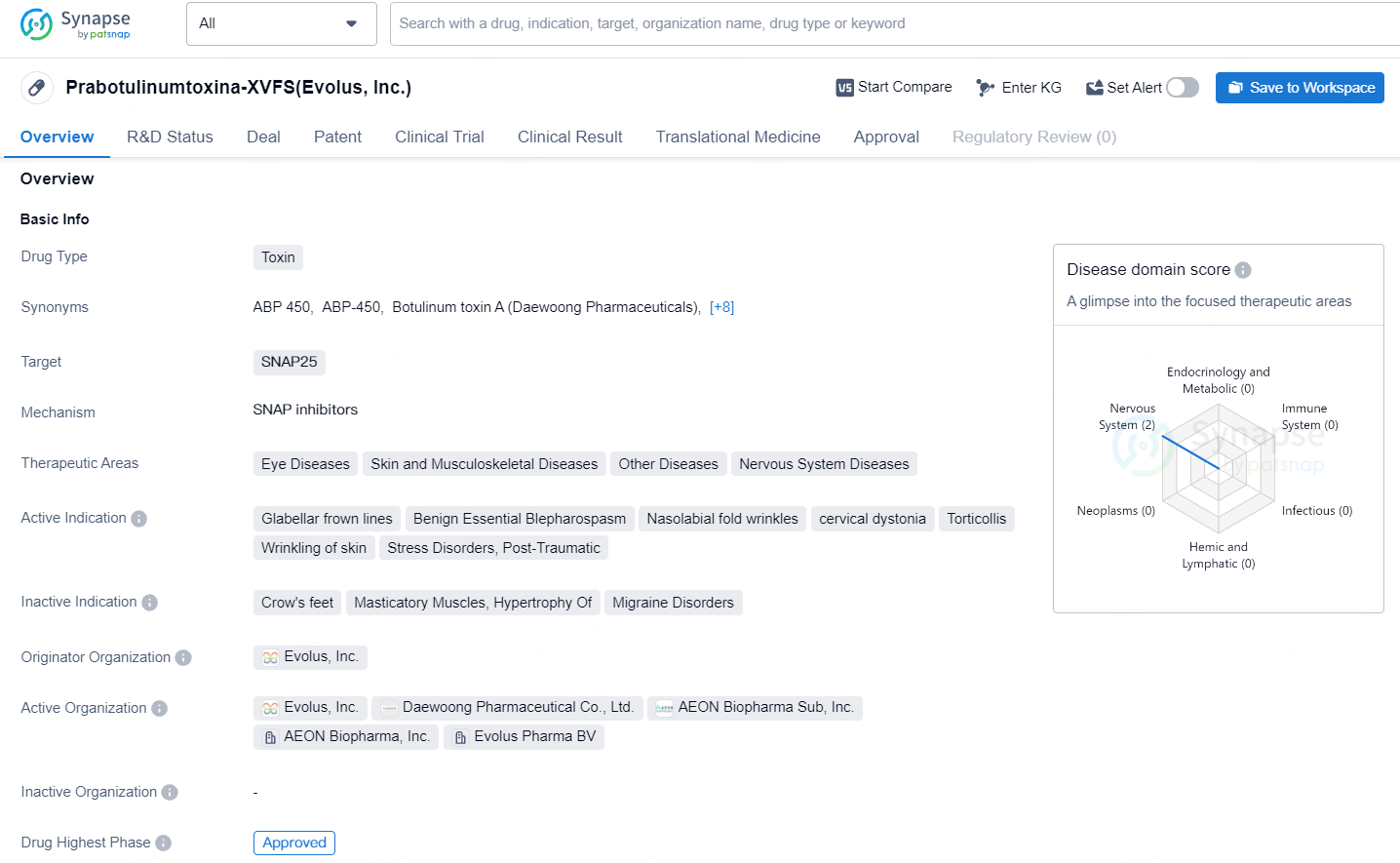
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In its submission to the FDA, AEON has presented comprehensive data derived from analytical, pharmacological, and animal studies, aiming to support a Biologics License Application. Success in a Phase 3 comparative study concerning CD could potentially provide the clinical data required to demonstrate that ABP-450 is highly similar to the reference product for all eight currently approved—and any future—therapeutic indications.
"We are convinced that our biosimilar strategy presents a significant opportunity for us to introduce ABP-450 to the U.S. market with a single approval covering all of BOTOX’s present and prospective therapeutic indications through conducting one comparative study," stated Marc Forth, AEON’s President and CEO. "We are eager to discuss our strategy with the FDA during a Biosimilar Initial Advisory Meeting scheduled for the third quarter."
ABP-450 has an identical 900kDa molecular weight to BOTOX. According to the 351 biosimilar regulatory pathway, the Company plans to leverage extensive analytical and preclinical testing, much of which has been completed. Post the FDA meeting in the third quarter of 2024, the Company anticipates proceeding with a Phase 3 CD study involving roughly 400 patients to directly compare ABP-450 to BOTOX.
AEON revealed data from its Phase 2 clinical trial of ABP-450 for CD treatment in September 2022, with further results presented at the International Parkinson and Movement Disorders Society Congress in August 2023.
Patients were monitored for up to 20 weeks, with the primary efficacy endpoint evaluated four weeks post-dosing. The dosing, tailored to each patient by the investigator, depended on factors such as the severity of the patient’s head and neck position, pain localization, muscle hypertrophy, patient response, and adverse event history.
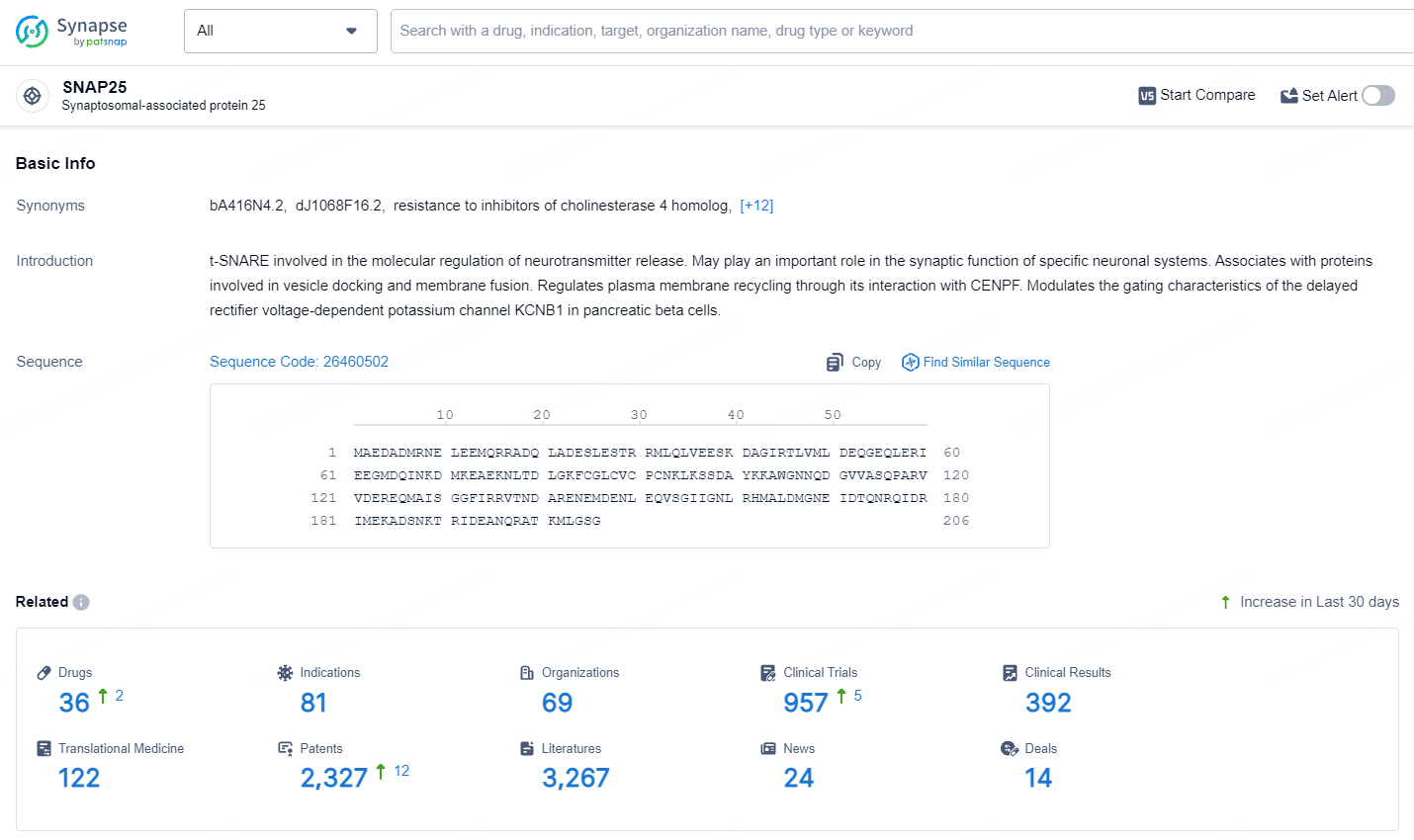
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 36 investigational drugs for the SNAP25 target, including 81 indications, 69 R&D institutions involved, with related clinical trials reaching 957, and as many as 2327 patents.
Prabotulinumtoxina-XVFS targets SNAP25 and is used in the treatment of various therapeutic areas. It has been approved for use in South Korea since 2013 and is currently in the highest phase of approval globally, with ongoing review in China. The drug's diverse therapeutic indications and approval status make it a significant asset in the pharmaceutical industry.