Is Myfembree approved by the FDA?
Myfembree is a combination oral medication containing estradiol, norethindrone, and relugolix. Myfembree was approved by the FDA on May 26, 2021. This approval allows it to be prescribed for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women, marking a significant step forward in treatment options for this condition .
Uses and Administration
Myfembree is specifically indicated for:
- Managing heavy menstrual bleeding associated with uterine leiomyomas (fibroids) in premenopausal women.
- Managing moderate to severe pain associated with endometriosis in premenopausal women.
The medication is taken orally, usually once a day, starting as early as possible after the onset of menses but no later than 7 days after menses has started. Treatment duration is typically limited to 24 months due to potential risks, including irreversible bone loss.
Side Effects and Precautions
Common side effects include:
- Hot flashes
- Unusual vaginal bleeding
- Decreased interest in sex
Serious side effects requiring immediate medical attention may include:
- Signs of a heart attack or stroke (chest pain, numbness or weakness, slurred speech)
- Severe allergic reactions (hives, difficulty breathing, swelling)
- Liver problems (nausea, vomiting, stomach pain, jaundice)
- New or worsening mental health issues (anxiety, depression, suicidal thoughts)
Patients using Myfembree are at an increased risk of blood clots, stroke, or heart attack, particularly if they smoke or are over 35 years old. It is crucial to discuss any pre-existing health conditions with a healthcare provider before starting Myfembree.
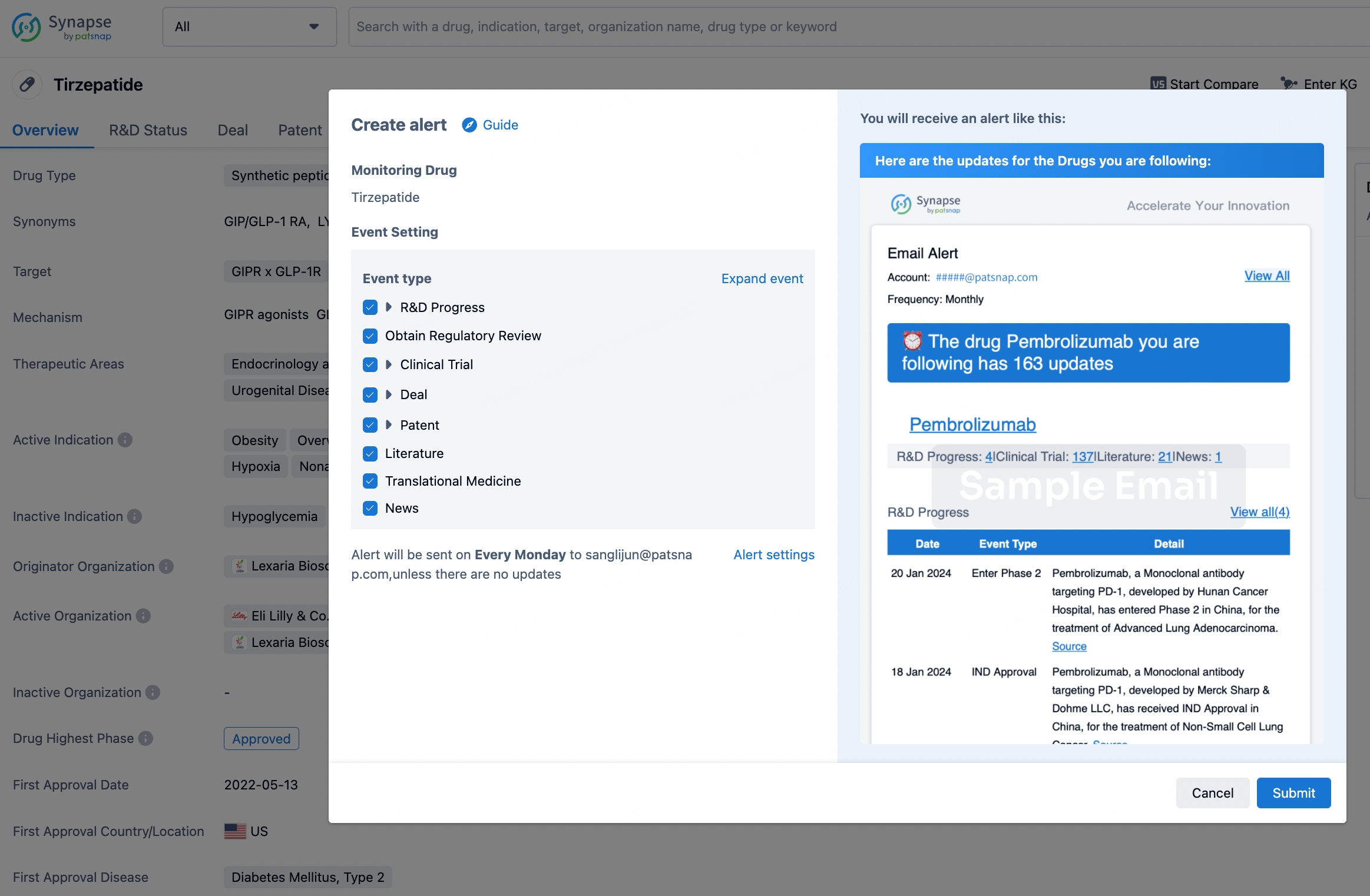
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!