Akeso's Cadonilimab and Ivonescimab Added to China's Reimbursed Drug List
Akeso, Inc. is excited to share that two of its self-developed, world-first bispecific antibody therapies-cadonilimab Injection (a PD-1/CTLA-4 bispecific antibody) and ivonescimab Injection (a PD-1/VEGF bispecific antibody)-have been added to the latest National Reimbursement Drug List (NRDL) published by the National Healthcare Security Administration of China.
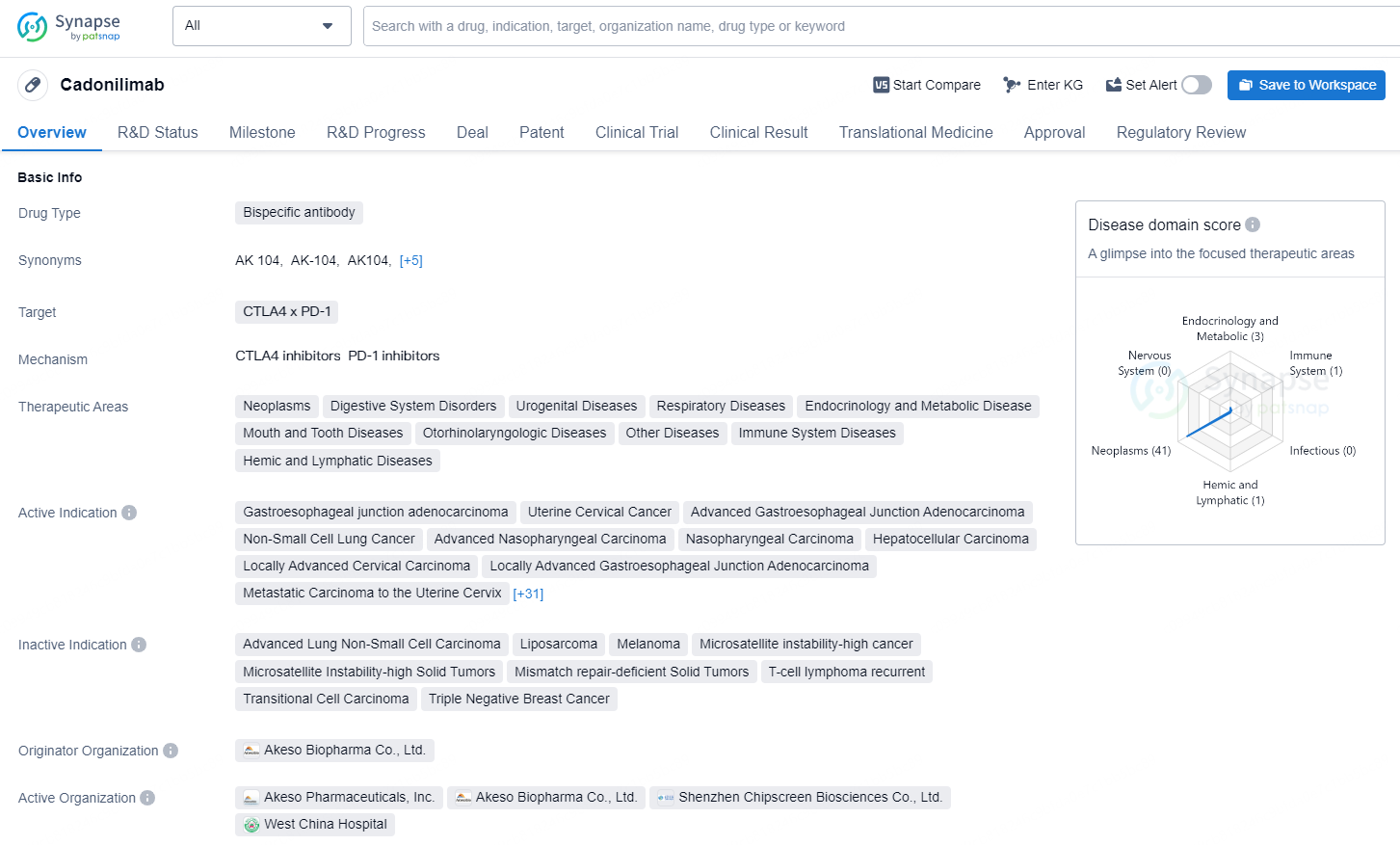
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Cadonilimab is approved for patients with relapsed or metastatic cervical cancer (R/M CC) who have shown disease progression following platinum-based chemotherapy.
China’s healthcare insurance framework comprises basic medical insurance, work-related injury coverage, and maternity insurance, ensuring access for over 95% of its citizens. The National Reimbursement Drug List (NRDL) specifies medications that qualify for reimbursement from the medical insurance fund. Statistical data indicates that in 2023, spending from the basic medical insurance, work-related injury insurance, and maternity insurance on drugs included in the NRDL constituted 90% of the total procurement orders for hospitals.
Dr. Xia Yu, the founder, chairwoman, president, and CEO of Akeso, stated, "The addition of cadonilimab and ivonescimab to the National Reimbursement Drug List underscores the innovative and clinical importance of these revolutionary biologics developed by Akeso. This inclusion represents a key advancement in improving patient access to cutting-edge therapies. It will significantly alleviate the financial burden on patients and enable a larger number to benefit from leading global treatments. This progression is perfectly aligned with Akeso’s fundamental belief in enhancing public health through scientific and technological advancements."
Cadonilimab: The World’s First Approved Bispecific Antibody for Cancer Immunotherapy
Cadonilimab is a pioneering bispecific antibody designed to target PD-1 and CTLA-4 concurrently, utilizing a synergistic approach to enhance antitumor efficacy. This distinctive mechanism markedly improves therapeutic results, delivering enhanced effectiveness while minimizing toxicity. Recent data on cadonilimab for R/M CC indicate that it is beneficial for both PD-L1 positive and negative patient groups, particularly for PD-L1 negative individuals who previously faced a lack of effective treatment options. In the context of R/M CC, cadonilimab achieved a median overall survival (mOS) exceeding 18 months (not reached), with an objective response rate (ORR) of 31.3% and a complete response (CR) rate of 13.1%. In the subgroup of PD-L1 positive patients, the ORR was 43.8%, along with a median progression-free survival (mPFS) of 6.34 months; the mOS remains to be determined.
As of September 2024, cadonilimab received approval from the National Medical Products Administration (NMPA) for use as a first-line treatment for advanced gastric cancer. The supplemental New Drug Application (sNDA) for its first-line use in advanced cervical cancer is currently under evaluation. Cadonilimab has been prominently recommended in 16 clinical treatment protocols and consensus statements, encompassing multiple cancer types, including gastric, gynecological, liver, esophageal, and nasopharyngeal cancers. Moreover, cadonilimab is involved in over 23 clinical trials across 16 different indications, which include gastric, lung, liver, cervical, and pancreatic cancers, with 8 of those being Phase III registration trials.
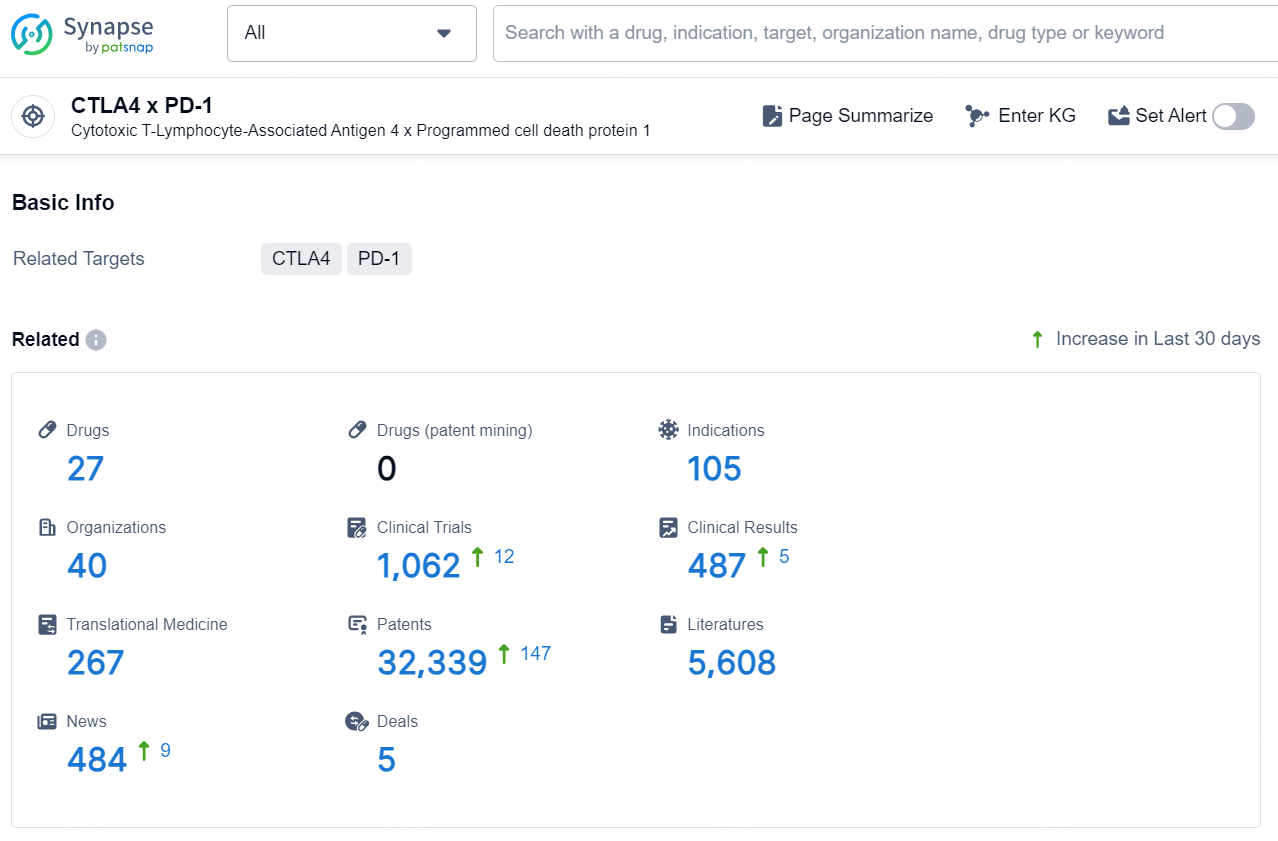
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 28, 2024, there are 27 investigational drugs for the CTLA4 x PD-1 target, including 105 indications, 40 R&D institutions involved, with related clinical trials reaching 1062, and as many as 32339 patents.
The drug Cadonilimab is a bispecific antibody that targets CTLA4 x PD-1, and it has been approved for use in the treatment of a wide range of therapeutic areas. These areas include neoplasms, digestive system disorders, urogenital diseases, respiratory diseases, endocrinology and metabolic disease, mouth and tooth diseases, otorhinolaryngologic diseases, immune system diseases, hemic and lymphatic diseases, and other diseases.