LEQEMBI®: New Alzheimer's Treatment Launched in South Korea
Eisai Co., Ltd. and Biogen Inc. announced the introduction of their humanized anti-soluble aggregated amyloid-beta (Aβ) monoclonal antibody "LEQEMBI®" in South Korea. The Ministry of Food and Drug Safety (MFDS) granted approval for LEQEMBI in May 2024, allowing its use for adult patients experiencing mild cognitive impairment associated with Alzheimer's disease (AD) or mild Alzheimer's dementia (early AD).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
LEQEMBI has a targeted affinity for both soluble amyloid beta (Aβ) aggregates, known as protofibrils*, and insoluble Aβ aggregates (fibrils), which are significant constituents of Aβ plaques in Alzheimer’s disease (AD). This binding action contributes to a reduction in both Aβ protofibrils and plaques within the brain. It is the first authorized therapy that has demonstrated a capability to decrease the progression rate of the disease and mitigate cognitive and functional deterioration through this approach.
In South Korea, it was estimated that in 2021 around 900,000 individuals were living with dementia, with one in ten people aged 65 and older affected by this condition, and one in five experiencing mild cognitive impairment (MCI). Reports indicate that Alzheimer’s dementia accounts for about 70% of the total dementia cases. The estimated average annual costs for care and treatment per dementia patient amount to approximately 21.1 million South Korean Won (KRW), while those with severe dementia incur costs up to 33.1 million KRW.
Eisai is at the forefront of LEQEMBI's global development and regulatory processes, with both Eisai and Biogen collaborating in the commercialization and promotion of this drug, while Eisai retains ultimate decision-making authority. In South Korea, distribution and informational activities for the product will be handled by Eisai Korea Inc.
Eisai Korea Inc. has long been a leader in dementia research and advocacy, concentrating on initiatives aimed at increasing awareness of the disease. Recently, they have partnered with a diverse range of stakeholders, including healthcare professionals, academic institutions, patient advocacy groups, care facilities, health screening companies, and diagnostic organizations, to cultivate a supportive ecosystem for dementia that fosters awareness and early intervention in AD. The initial launch of the medication will occur in the private sector, alongside the implementation of a Patient Assistance Program, aimed at ensuring access to lecanemab for patients in need, with the goal of positively influencing patients, their families, and society as a whole in South Korea.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
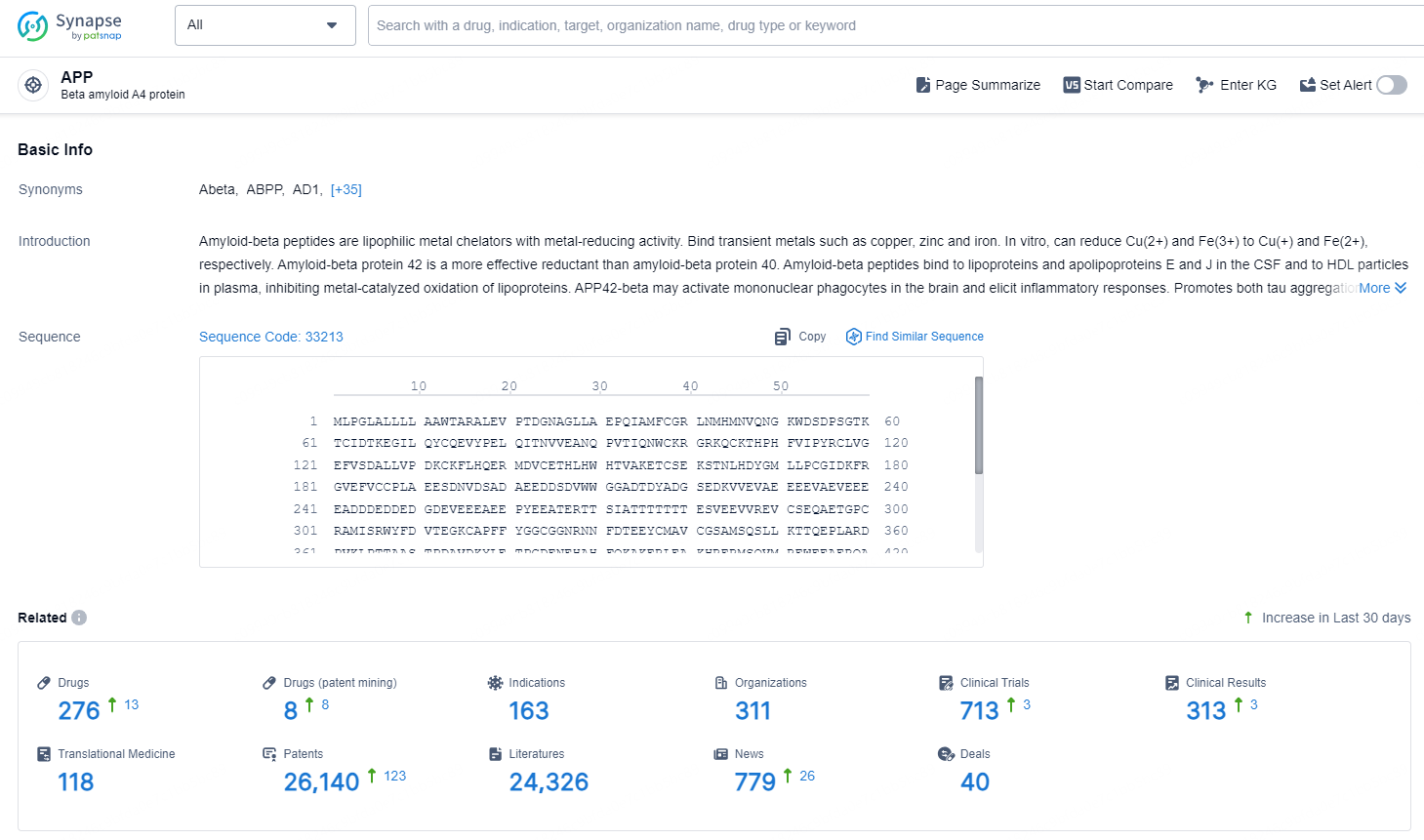
According to the data provided by the Synapse Database, As of November 28, 2024, there are 276 investigational drugs for the APP target, including 163 indications, 311 R&D institutions involved, with related clinical trials reaching 713, and as many as 26140 patents.
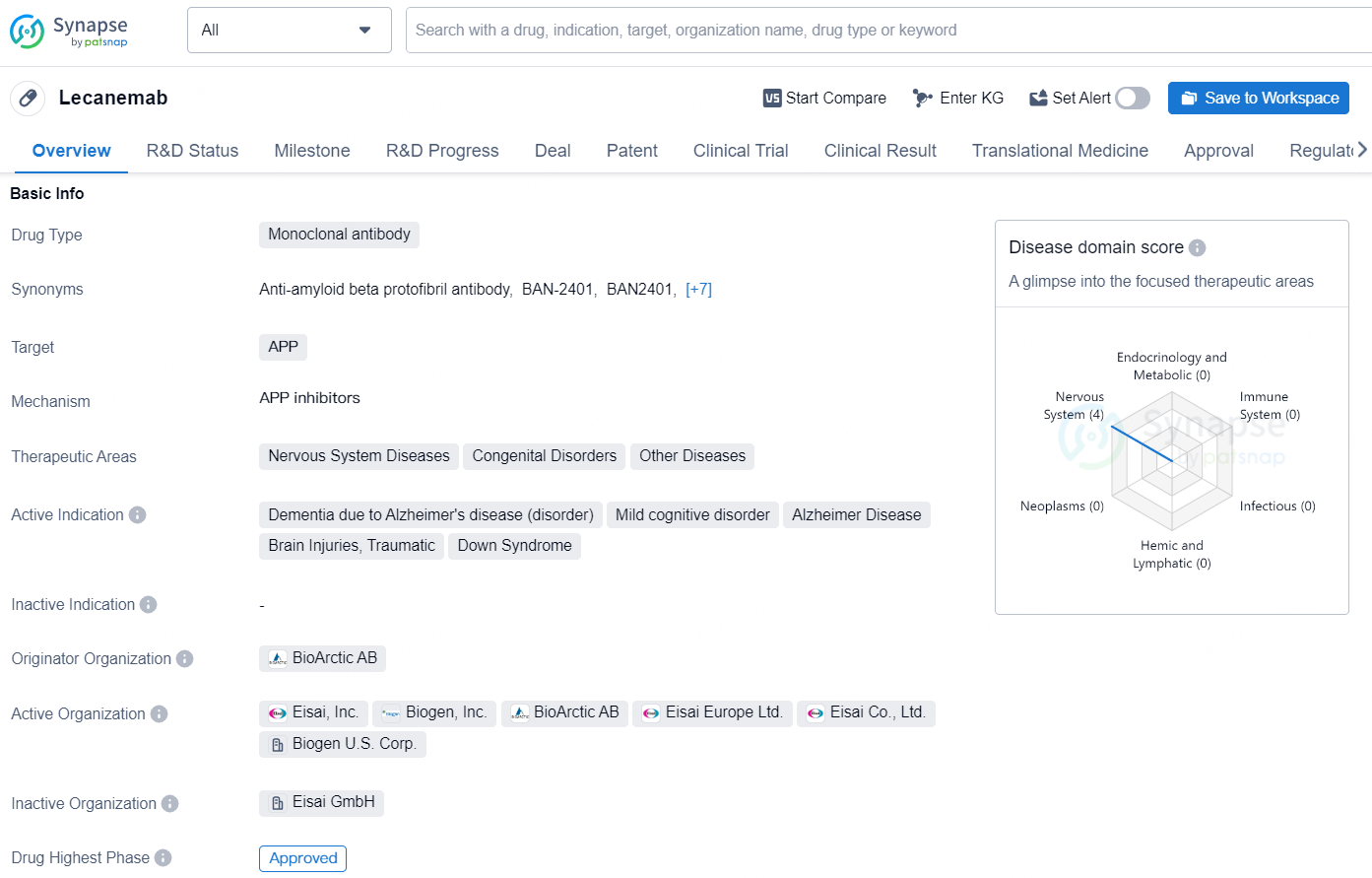
Lecanemab is a monoclonal antibody drug developed by the Originator Organization, BioArctic AB. It primarily targets Amyloid Precursor Protein (APP) and is indicated for the treatment of various conditions related to nervous system diseases, congenital disorders, and other diseases. The drug has received its highest global and China phase approvals and was first approved in the United States in January 2023.