ALETA-001 Trial Begins: Targeting Recurrent B-cell Cancers
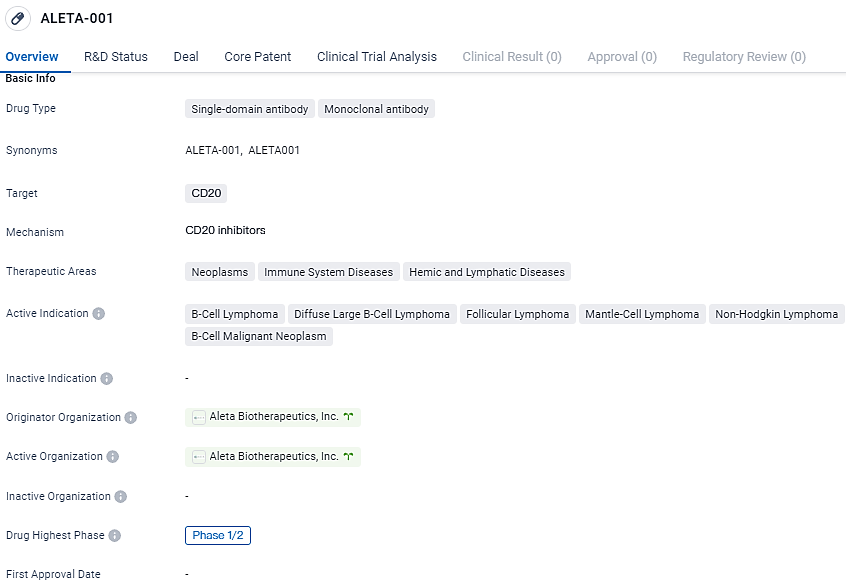
Aleta Biotherapeutics, an enterprise operating in the clinical phase and specializing in immuno-oncology, utilizes a proprietary CAR T-Cell Engager technology to enhance the performance of cellular cancer treatments. In collaboration with the Centre for Drug Development at Cancer Research UK, they have proclaimed the initiation of dosing in a patient as part of a clinical trial at the Phase 1/2 level.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Currently underway, this study focuses on the pioneering biopharmaceutical agent, ALETA-001, developed to address B-cell malignancies in patients wherein previous CD19-specific CAR T-cell therapy has been unsuccessful. The Centre for Drug Development at Cancer Research UK is both the sponsor and the principal investigator for the initial two phases of clinical trials for ALETA-001, which stands as the primary compound within Aleta Biotherapeutics' range of products.
A milestone in clinical research, the move to study ALETA-001 in a medical setting marks a profound advancement in cancer therapy. "Witnessing ALETA-001 in clinical trials is an inspiring development. The examination of ALETA-001's ability paves the way towards creating an alternative therapeutic avenue for numerous patients who no longer respond to their existing CD19-focused CAR T-cell treatments. Our hope is that ALETA-001 will both replenish and magnify the CAR T-cells' capability to eradicate cancerous cells, thus proposing an improved outcome for individuals undergoing cell-based treatments," announced Dr. Paul Rennert, who leads as the President and Chief Scientific Officer at Aleta Biotherapeutics.
The Phase 1/2 trial of ALETA-001 is structured as an open-label study to advance dosage levels, determined to assess not only the safety and tolerability of the compound but also to examine the pharmacological kinetics and dynamics, alongside preliminary effectiveness indicators in isolation.
Dr. Lars Erwig, who holds the position of Director of Drug Development at Cancer Research UK, remarked, "An unfortunate reality for many patients suffering from hematologic cancers is the potential for regression post-treatment with CD19-centric CAR T-cell therapies. At Cancer Research UK, the potential ALETA-001 demonstrates to revolutionize and potentially save lives through its impact on treatment narratives, gives us a great deal of hope for the future."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
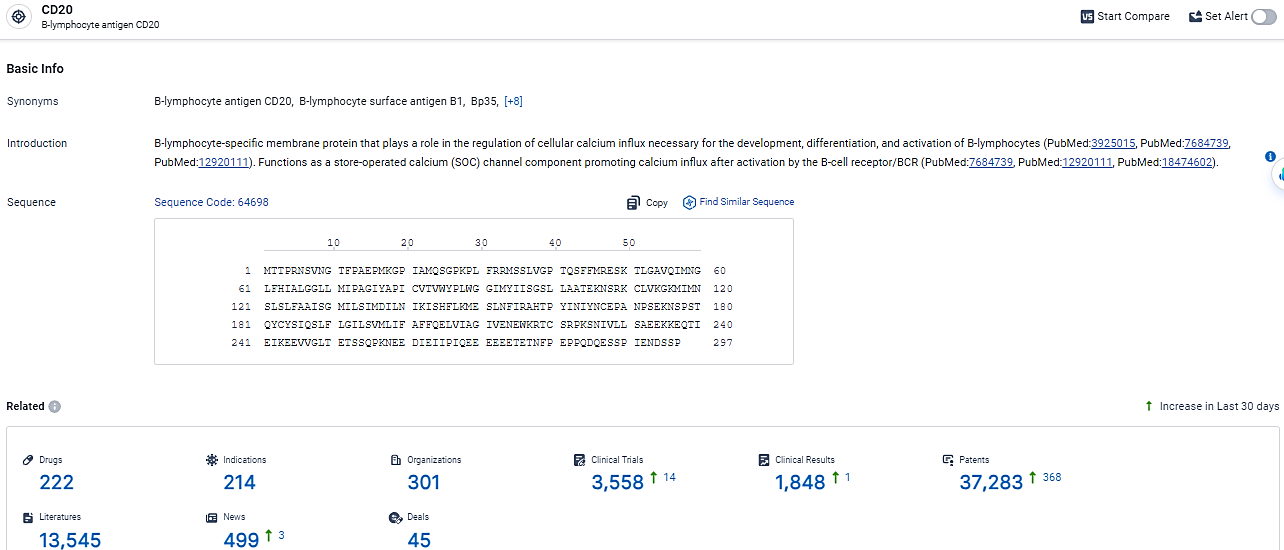
According to the data provided by the Synapse Database, As of February 26, 2024, there are 222 investigational drugs for the CD20 target, including 214 indications,301 R&D institutions involved, with related clinical trials reaching 2558, and as many as 37283 patents.
ALETA-001 targets CD20 and is being investigated for its potential in treating various B-cell lymphomas. Currently in Phase 1/2 of clinical development, the drug has been granted orphan drug status. Further research and clinical trials will be necessary to determine its safety and efficacy in larger patient populations.