Alvotech, Teva Gain FDA Approval for Stelara® Biosimilar SELARSDI™
Alvotech, in collaboration with Teva Pharmaceuticals—an American affiliate of Teva Pharmaceutical Industries Ltd.—has announced the approval of SELARSDI (ustekinumab-aekn) by the U.S. Food and Drug Administration. This subcutaneously injected biosimilar, comparable to Stelara, is authorized for managing moderate to severe plaque psoriasis and active psoriatic arthritis in adults, as well as in pediatric patients aged 6 and above. Under the terms of their strategic agreement, Teva will exclusively handle the commercial distribution of SELARSDI within the United States.
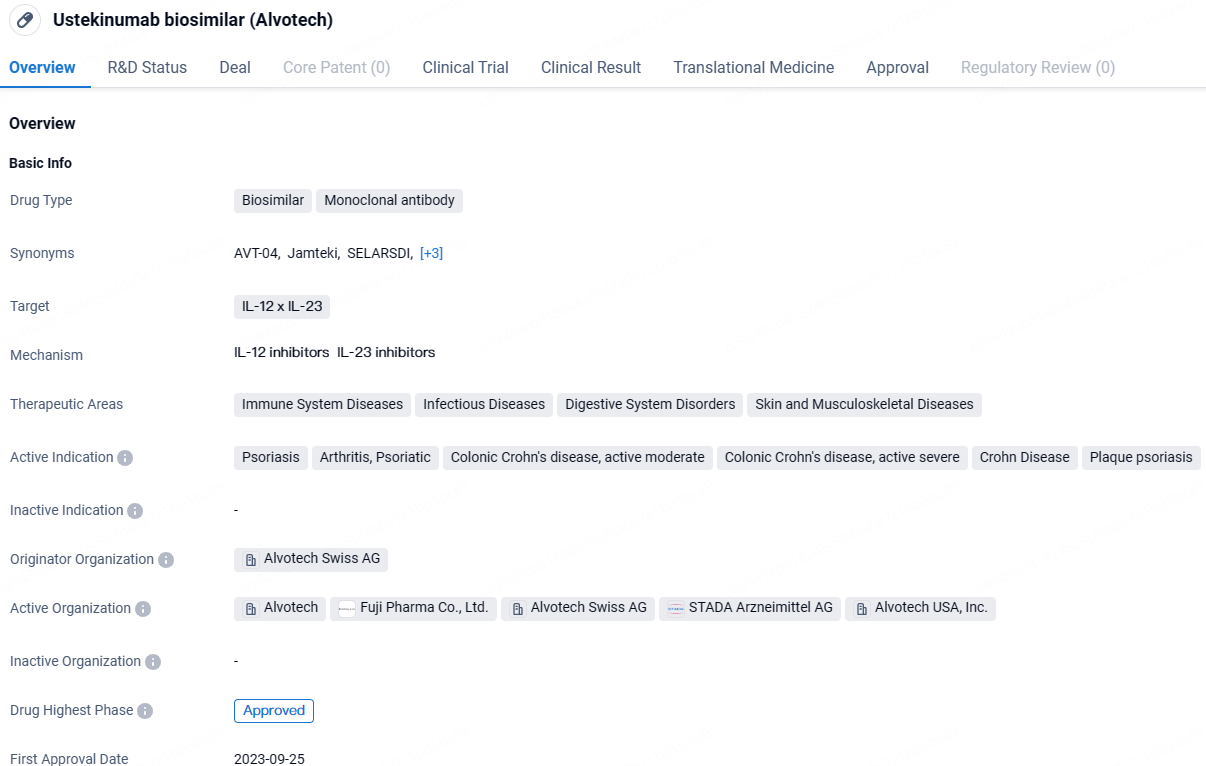
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Thomas Rainey, the Senior Vice President of U.S. Market Access at Teva, highlighted the significance of acquiring the second biosimilar endorsement this year with SELARSDI, stressing Teva's dedication to increasing availability and enhancing patient access to these key treatment choices within the United States. "The biosimilar sector is expanding both globally and domestically, and these products are crucial to Teva's growth-focused strategy," Rainey announced. He also mentioned the strategic partnerships that enhance Teva's ability to utilize its global commercial experience and push for further biosimilar introductions to the market.
Ustekinumab, which targets the p40 subunit of IL-12 and IL-23 cytokines, is utilized in therapies for immune-mediated conditions like psoriasis and psoriatic arthritis. Alvotech uses Sp2/0 cells via a continuous perfusion method for producing SELARSDI, mirroring the production techniques of the reference biologic, Stelara.
The U.S. sales for Stelara nearly reached $7 billion in 2023. The introduction of SELARSDI as a biosimilar to Stelara is expected to foster notable healthcare savings and expand the range of therapeutic options available for treating diseases. Generally, plaque psoriasis is the most prevalent type of psoriasis in the U.S., with psoriatic arthritis representing about six percent of juvenile arthritis cases.
In June 2023, Alvotech and Teva settled with the original biologic's manufacturer, Johnson & Johnson, securing a license for SELARSDI in the U.S. that becomes effective no later than February 21, 2025.
Back in August 2020, a strategic tie between Alvotech and Teva was established for the exclusive marketing of five biosimilar candidates from Alvotech. This alliance expanded in August 2023 to include two more biosimilars and new variants of two existing products. Alvotech remains in charge of development and manufacturing, while Teva handles exclusive commercialization tasks in the United States, benefitting from its robust market presence and marketing capabilities. Notably, SELARSDI is the second biosimilar approved through this collaboration, following the FDA's approval in February 2024 of SIMLANDI®, a citrate-free, high-concentration biosimilar to Humira that has also been granted interchangeability status by the FDA.
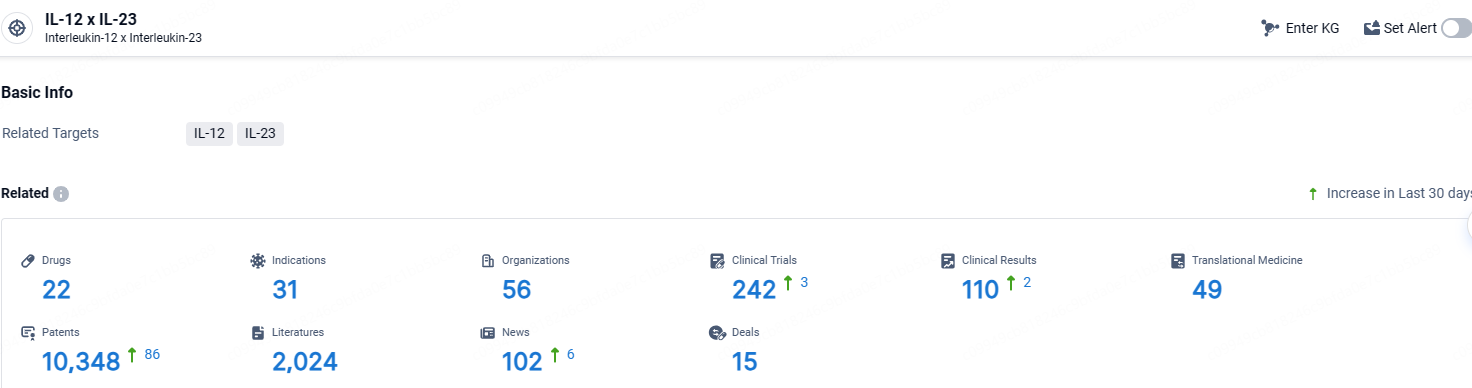
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 18, 2024, there are 22 investigational drugs for the IL-12 and IL-23 target, including 31 indications, 56 R&D institutions involved, with related clinical trials reaching 242, and as many as 49 patents.
ustekinumab-aekn is a promising monoclonal antibody drug that targets IL-12 x IL-23 proteins. It has shown potential in treating various immune system, infectious, digestive, and musculoskeletal disorders. While it has received global approval and is expected to be available in Japan by September 2023, its approval in China is still pending. This drug represents an important development in the field of biomedicine and has the potential to significantly improve the lives of patients suffering from the indicated conditions.