Eloxx Pharmaceuticals Offers Updates on ELX-02 and ZKN-013 Developments
Eloxx Pharmaceuticals, Inc., a forefront company in the development of genetic treatments targeting ribosomal RNA for rare diseases, announced recent developments in their programs for ELX-02 and ZKN-013, highlighting the Orphan Drug Designation awarded to ELX-02.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
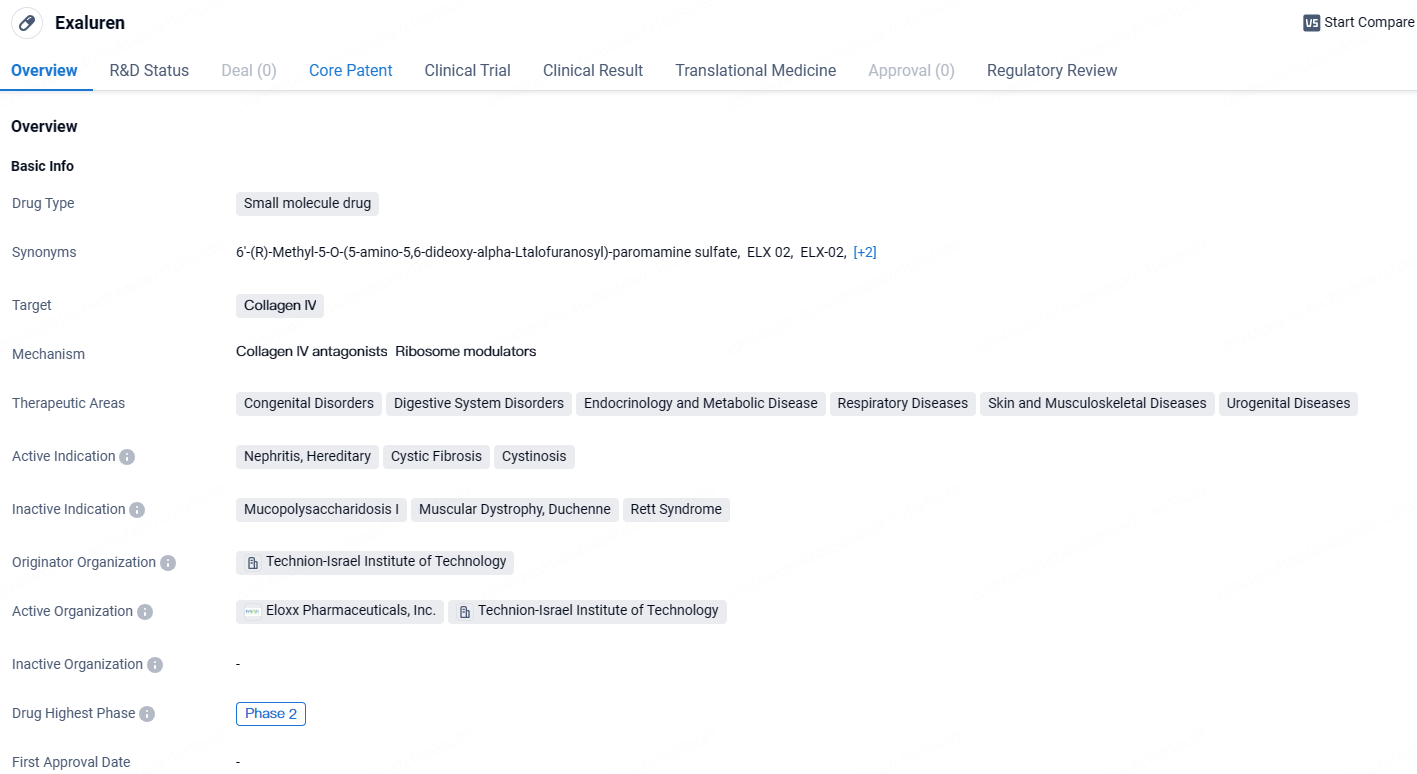
Eloxx has made considerable strides in the progress of ELX-02 aimed at treating Alport syndrome characterized by Nonsense Mutations. Furthermore, following a worldwide licensing agreement with Almirall that began in March, Eloxx initiated the research on ZKN-013 for addressing Recessive Dystrophic Epidermolysis Bullosa and Familial Adenomatous Polyposis in patients with nonsense mutations.
A recent study conducted on Autosomal Dominant Polycystic Kidney Disease has solidified ELX-02’s potential as a viable treatment for various rare genetic kidney diseases where nonsense mutations are present in the causative genes.
”The latest updates to the ELX-02 program, including receiving Orphan Drug Designation for its application in Alport Syndrome, underscore the critical medical requirements of the patients afflicted with Nonsense Mutations,” remarked Sumit Aggarwal, President and CEO of Eloxx.
He further emphasized, “This recognition enhances our confidence that ELX-02 could play a transformative role for the specially targeted Alport Syndrome patients having nonsense mutations. We are eager to begin discussions with the FDA to launch a comprehensive clinical trial, aiming to secure regulatory marketing authorization for ELX-02 for these patients.”
The FDA grants Orphan Drug Designation to drugs that propose treatment for rare disorders impacting less than 200,000 Americans, supported by a solid clinical or preclinical rationale that they will be effective in the intended population.
Eloxx also requested a Pre-Investigational New Drug meeting with the U.S. FDA: This pre-IND meeting request marks the commencement of formal discussions with the FDA concerning the development of ELX-02 for treating NMAS. The primary goal of this meeting is to obtain feedback on Eloxx’s proposed clinical trials for Alport syndrome patients with nonsense mutations.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
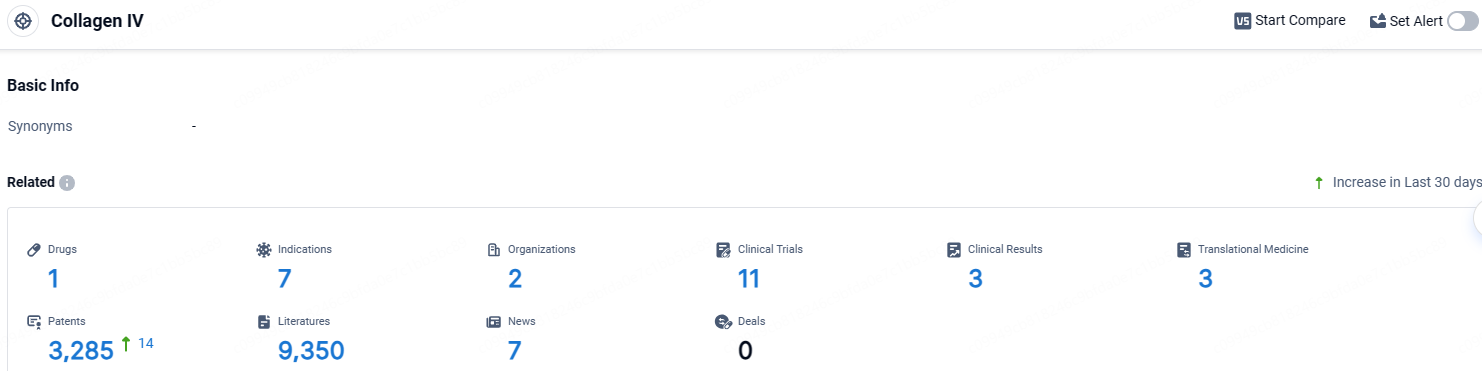
According to the data provided by the Synapse Database, As of April 18, 2024, there are 1 investigational drugs for the Collagen IV target, including 7 indications, 2 R&D institutions involved, with related clinical trials reaching 11, and as many as 3 patents.
Exaluren is a small molecule drug that targets Collagen IV and is being developed for the treatment of various therapeutic areas including congenital disorders, digestive system disorders, endocrinology and metabolic disease, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases. It has shown promise in treating nephritis, hereditary, cystic fibrosis, and cystinosis. The drug has been granted Fast Track and Orphan Drug designations, highlighting its potential to address unmet medical needs and rare diseases.