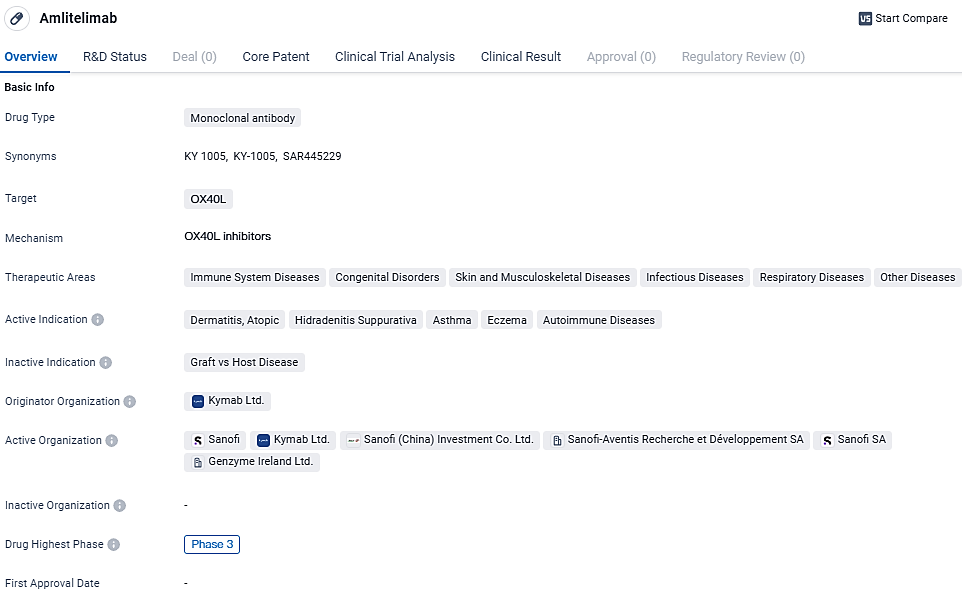
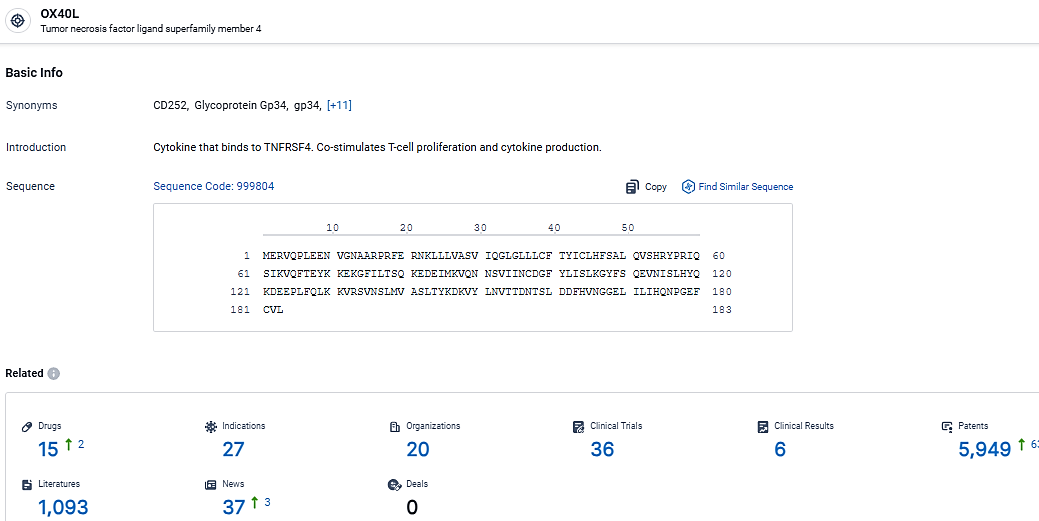
Amlitelimab Shows Promise as a Leading Long-term Eczema Treatment: Phase 2 Results
Encouraging findings emerged from the second segment of the STREAM-AD Phase 2b trial evaluating amlitelimab, wherein adults exhibiting moderate to severe atopic dermatitis (AD) and having previously shown responsiveness to amlitelimab displayed continuous amelioration in clinical manifestations for a duration of 28 weeks upon maintaining therapy. Additionally, a significant proportion of participants who discontinued the use of amlitelimab also demonstrated favorable response rates.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The tolerability of amlitelimab maintained alignment with the initial phase of research, showing no emergent safety concerns while continuing to be well-received. These findings were showcased during a prominent late-breaking session at the American Academy of Dermatology's 2024 gathering in San Diego, which further bolstered the rationale for exploring a three-month dosing interval of amlitelimab, comprising an initial 500 mg loading dosage followed by 250 mg thereafter. The ongoing and broader Phase 3 study is currently examining this regimen.
"Insights gathered from the recent data suggest that amlitelimab may retain its effects even after the cessation of treatment, justifying the ongoing assessment of a quarterly dosing regimen. This has the potential to significantly ease the treatment regimen for those affected by AD," stated Professor Stephan Weidinger, M.D., Ph.D., leading expert and head of the Dermatology and Allergy department at the University Hospital Schleswig-Holstein.
In both the patient cohorts that discontinued and those that maintained their amlitelimab treatments, levels of biomarkers associated with AD were observed to be lower at the 52-week mark, even though amlitelimab levels in the bloodstream reached minimal levels. Benchmarks like TARC, eosinophil counts, and IL-22 that had reduced by the 24-week mark remained low throughout the no-treatment phase and amongst patients who continued with the therapy up to the 52-week mark. The consistency of these biomarker levels indicates a lasting modulation of inflammatory T cells through OX40L inhibition and the sustained management of AD even after stopping amlitelimab.
Amlitelimab stands out as a fully human monoclonal antibody that selectively inhibits the OX40-Ligand without depleting T cells, thus positioning itself as a leading or potentially unrivaled therapeutic option for an array of immune-mediated and inflammatory conditions. These include not only AD but also asthma, hidradenitis suppurativa, scleroderma, celiac disease, and alopecia. Amlitelimab's mechanism focusing on OX40-Ligand is tailored to recalibrate the balance between the body's pro-inflammatory and regulatory T cells.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 12, 2024, there are 15 investigational drugs for the OX40L target, including 27 indications, 20 R&D institutions involved, with related clinical trials reaching 36, and as many as 5949 patents.
Amlitelimab aims to address a wide range of therapeutic areas, including immune system diseases, congenital disorders, skin and musculoskeletal diseases, infectious diseases, respiratory diseases, and other diseases. Its active indications include dermatitis, atopic, hidradenitis suppurativa, asthma, eczema, and autoimmune diseases. The specific targeting of OX40L suggests that Amlitelimab may have the potential to modulate the immune response and provide therapeutic benefits for patients suffering from these conditions.