Sensorion's Phase 2a Trial: SENS-401 Meets Primary Objective in Hearing Preservation
Sensorion, an innovative biopharmaceutical enterprise committed to crafting new treatments aimed at managing, reversing, and averting auditory impairments, has declared its success in reaching the main goal in a Phase 2a clinical trial evaluating the efficacy of SENS-401. This Proof of Concept trial was focused on maintaining the existing hearing capability of patients who have received cochlear implant surgeries.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Stage 2a investigation is a multi-center, open-label, controlled trial with randomization, aimed at determining the systemic availability of SENS-401 within the cochlea at levels considered therapeutic after a regimen of oral doses administered twice for seven days before a patient receives a cochlear implant due to significant to severe hearing loss.
Participants commenced the SENS-401 therapy seven days preceding their implant surgeries and continued the medication for an additional six weeks. This trial additionally monitors several secondary outcomes, such as the variation in hearing sensitivity from the initial baseline to the trial's conclusion in the ear receiving the implant, across a range of sound frequencies. Sensorion has partnered with Cochlear Limited, a leading entity in the field of implantable hearing solutions, for the development of this study.
On the first of February, 2024, Sensorion proclaimed the successful inclusion of all participants in the Stage 2a clinical assessment of SENS-401, which is focused on the preservation of hearing that remains post-cochlear implant procedure. There have been 28 participants randomized within this study, and from these, 25 have undergone cochlear implantation – with 16 in the group receiving SENS-401 treatment and 9 in the untreated control cohort.
The detection of SENS-401 within the perilymph, indicative of a potentially efficacious threshold, was verified in every patient evaluated seven days subsequent to initiation of therapy, signifying the primary objective of the trial was achieved. These findings substantiate the ability of orally consumed SENS-401 to permeate the barrier of the inner ear.
Although the study has officially come to an end, the monitoring of the final participants persists, and in-depth analyses of secondary objectives, including those pertaining to the maintenance of existing hearing, are slated for completion and examination later within the year. Sensorion has scheduled to reveal the comprehensive conclusion of this study in the third quarter of 2024.
In the year 2017, an agreement for collaborative research was formed between Sensorion and Cochlear focusing on SENS-401. Following this agreement, Cochlear secured the option to enter into negotiations with Sensorion for the acquisition of worldwide licensing rights to SENS-401 connected with particular implantable hearing devices, contingent upon the availability of the complete data from this study.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
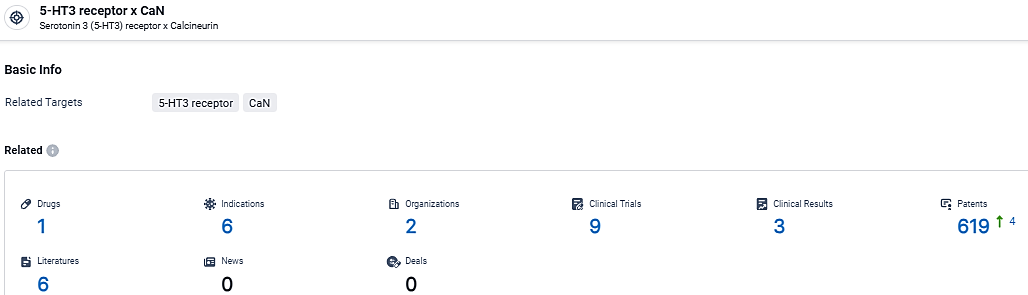
According to the data provided by the Synapse Database, As of March 11, 2024, there are 1 investigational drugs for the 5-HT3 receptor and CaN target, including 6 indications, 2 R&D institutions involved, with related clinical trials reaching 9, and as many as 619 patents.
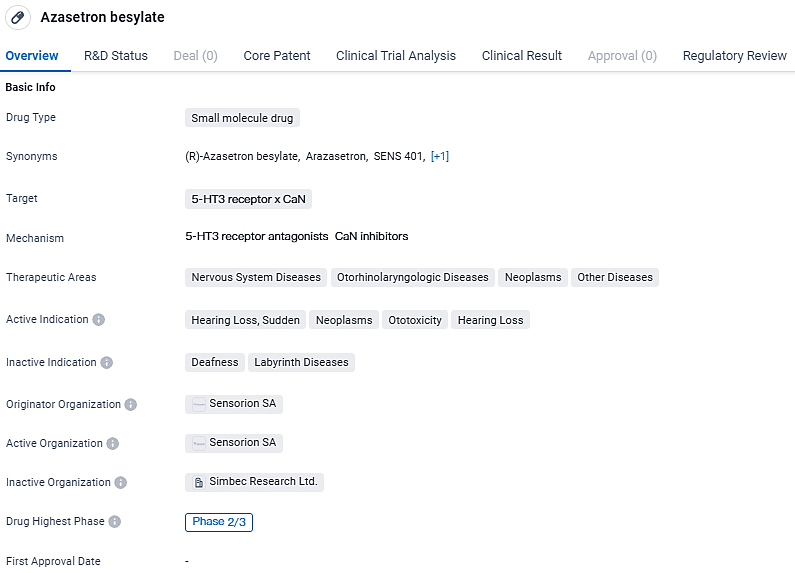
Azasetron besylate is a small molecule drug targeting the 5-HT3 receptor and CaN. The drug aims to address various diseases and conditions related to the nervous system, otorhinolaryngologic system, and neoplasms. Regulatory attention in the form of a Paediatric investigation plan and Orphan Drug designation further emphasizes the potential significance of Azasetron besylate in specific patient populations.