An analysis of IMM-27M's R&D progress and its clinical results presented at the 2024 AACR Annual Meeting
On April 5, 2024, the latest clinical findings of IMM27M were unveiled at the 2024 AACR, demonstrating its potential effect and setting the stage for subsequent investigations.
IMM-27M's R&D Progress
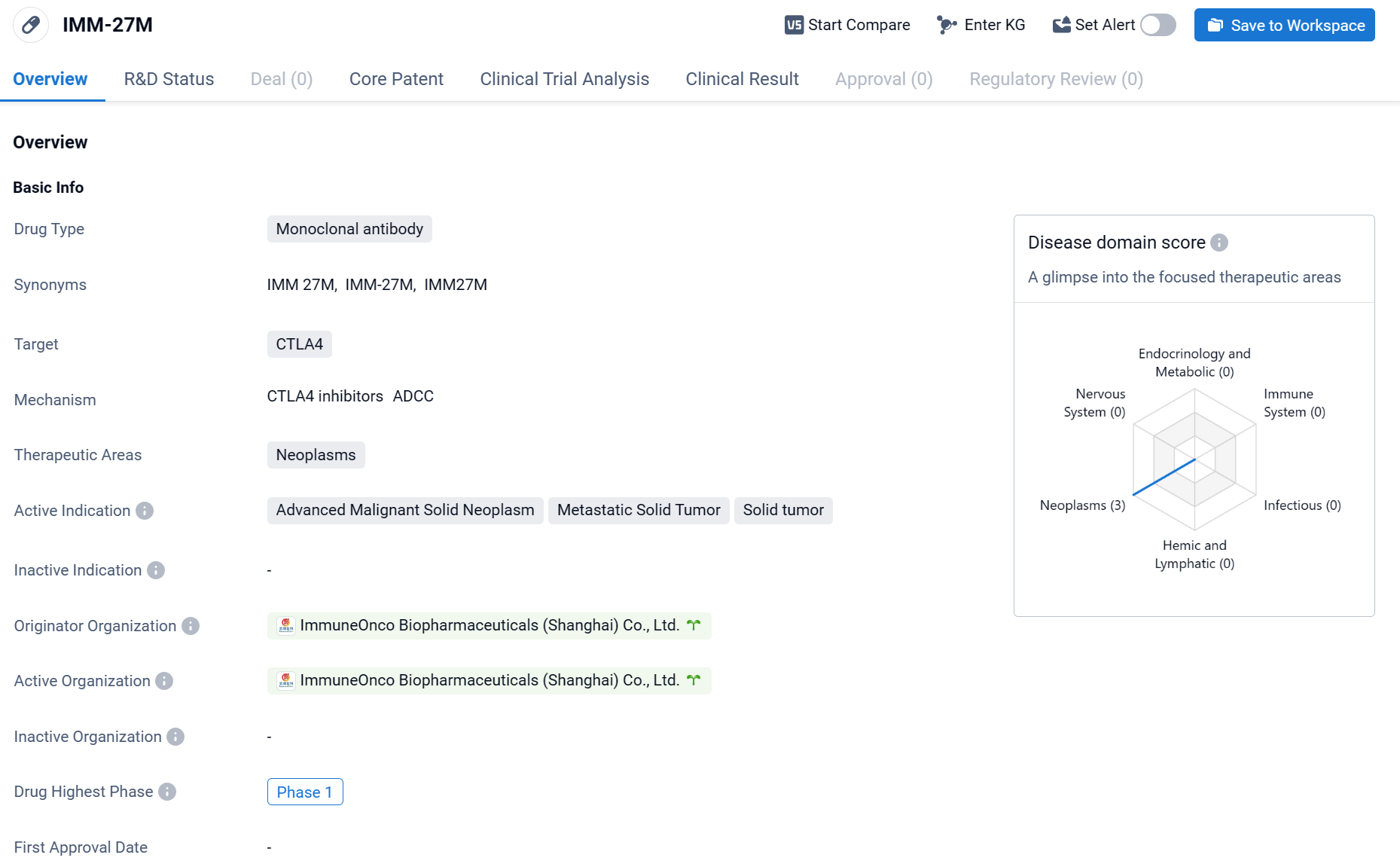
The drug IMM-27M is a monoclonal antibody that targets CTLA4, a protein involved in regulating the immune system. It is being developed by ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd. for the treatment of neoplasms, which are abnormal growths of tissue commonly referred to as tumors.
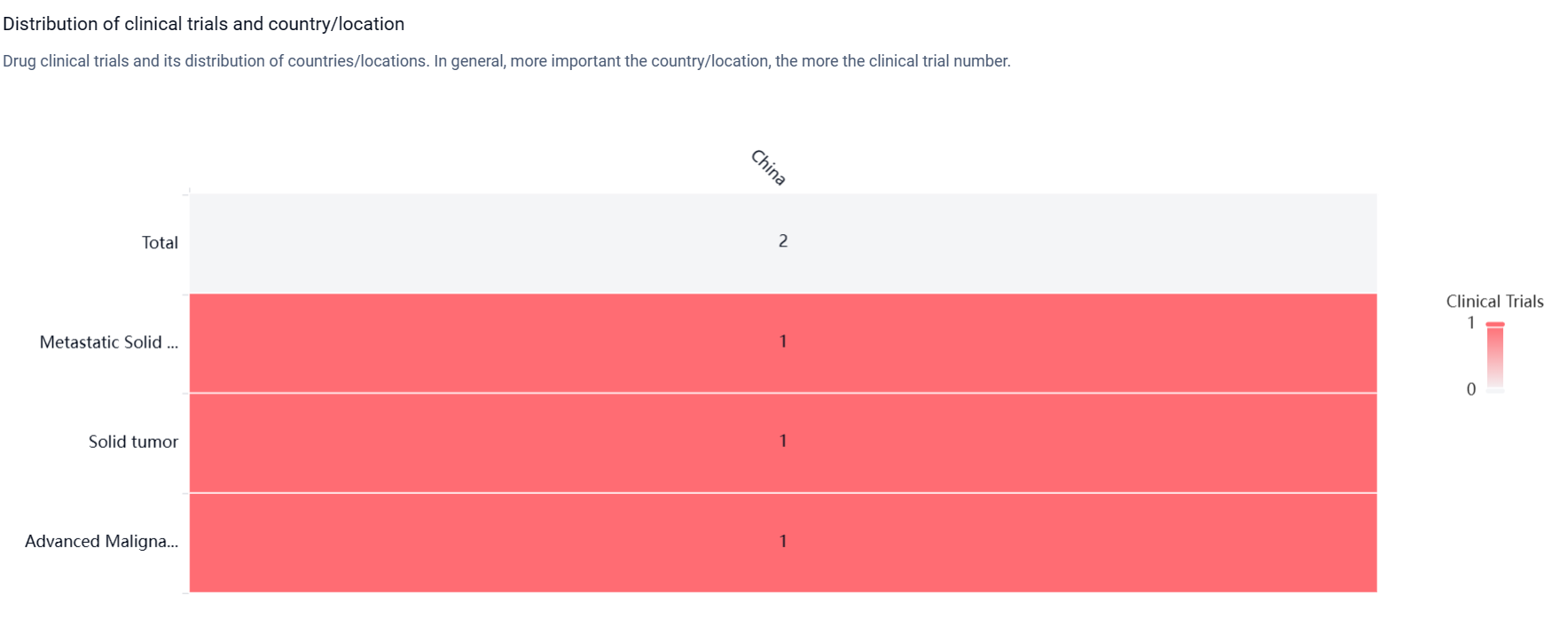
According to the Patsnap Synapse, IMM-27M is currently in Phase 1 of clinical development, which is the earliest stage of testing in humans. And the clinical trial distribution for IMM-27M is primarily in China. The key indication is Metastatic Solid Tumor.
Detailed Clinical Result of IMM-27M
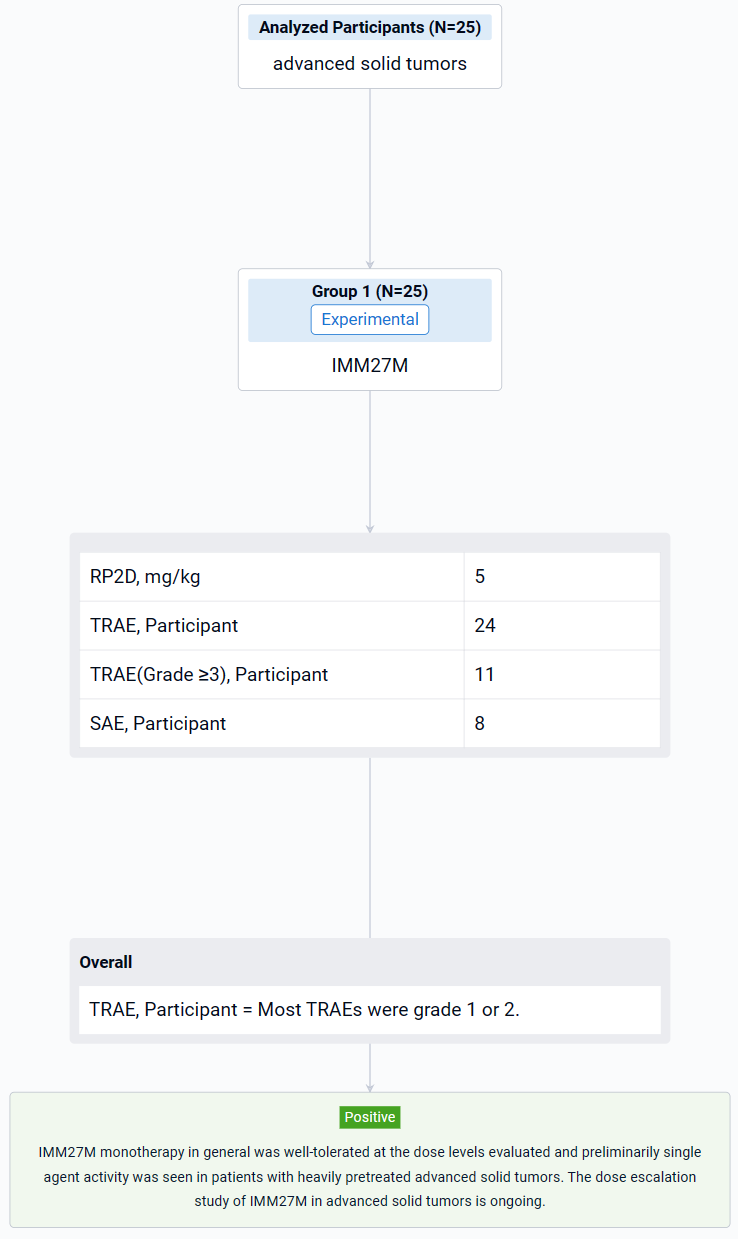
This study (NCT05235438) is an open-label, multi-center, phase I dose-escalation study to evaluate the safety, tolerability, maximum tolerated dose/recommended dose for expansion, PK and anti-tumor activity in patients with advanced solid tumors.
The study was designed with an accelerated titration followed by a standard 3+3 design. IMM27M (0.1, 0.3, 1.0, 2.0, 3.0, 5.0, 7.5, 10.0 mg/kg) was administered as monotherapy Q3W.
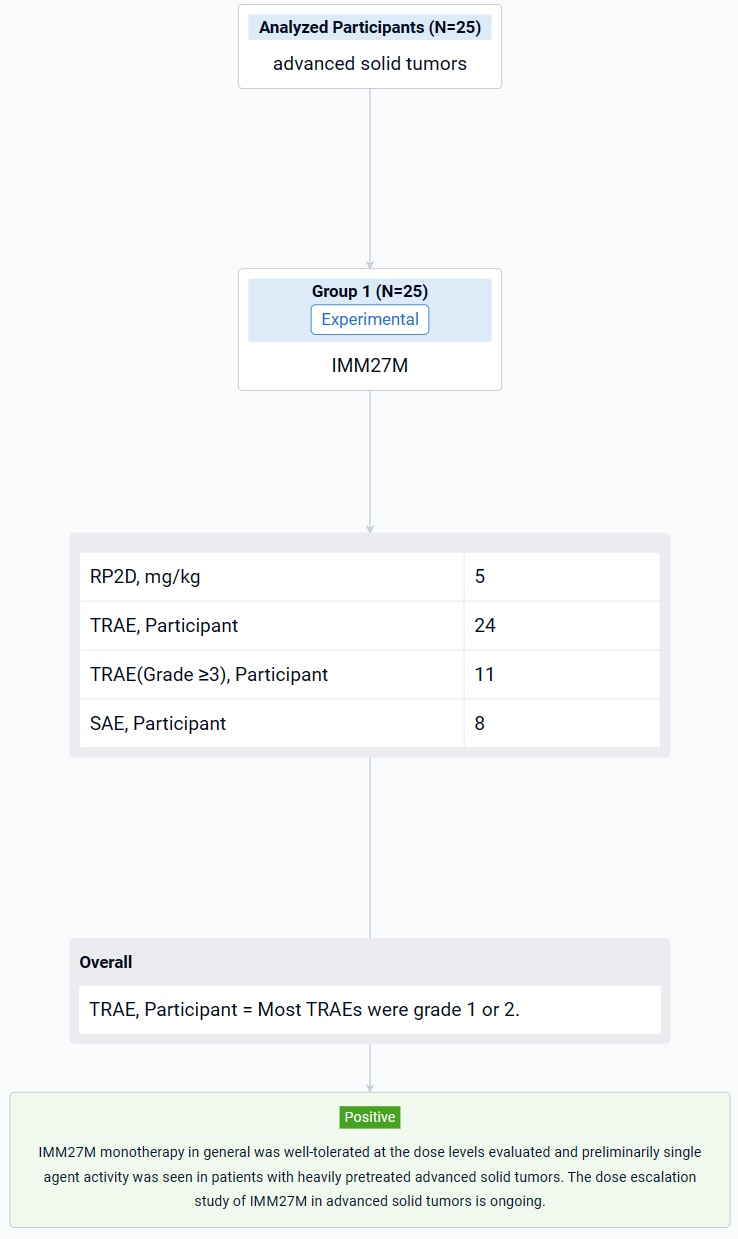
The result showed that as of 3 Nov 2023, the dose has been escalated to 7.5 mg/kg. 25 patients (20 females, 5 males) were enrolled and treated (1 at 0.1 mg/kg, 3 at 0.3 mg/kg, 3 at 1.0 mg/kg, 4 at 2.0 mg/kg, 4 at 3.0 mg/kg, 7 at 5.0 mg/kg, 3 at 7.5 mg/kg), including 13 patients with breast cancer (10 HR+ mBC), 4 patients with melanoma, 3 patients with RCC, each 1 patient with HCC, NSCLC and ovarian cancer, respectively. Median age was 51 years (range 31-72). 92.0% of the patients previously received ≥ 2 lines of systemic therapies and 52.0% received anti PD-1/PD-L1 treatment. Treatment-related adverse events (TRAEs) occurred in 24 patients (96.0%) by the data cutoff. Most TRAEs were grade 1 or 2. The most common TRAEs (≥ 30%) of all grades were anaemia (56.0%), lymphocyte count decreased (48.0%), aspartate aminotransferase increased (40.0%), hypoalbuminaemia (40.0%), decreased appetite (40.0%). Grade ≥3 TRAEs occurred in 11 patients (44.0%). Grade ≥3 TRAEs (≥10%) were lymphocyte count decreased (16.0%) and anaemia (12.0%). Treatment related SAE occurred in 8 patients (32.0%). No DLT was observed. One TRAE (Grade 3 immune related enteritis) led to treatment discontinuation. No TEAE leading to death was reported. Recommended Phase 2 Dose (RP2D) was 5 mg/kg. In 25 response evaluable patients, 2 patients had confirmed PR: 1 patient with mBC (HR+/HER2+, IO naïve, 6L previous treatments) at 3.0 mg/kg and response durable for 7 months; 1 patient with mBC (HR+/HER-, IO naïve, 4L previous treatments) at 5.0 mg/kg and response durable for 3 months by data cut-off. In addition, 9 patients had BOR SD and 4 out of 9 were previously treated with IO. Another 2 out of 8 patients with HR+ mBC had BOR SD with over 10% decreased tumor burden. IMM27M in vivo exposure increased with dose. The T1/2 mean value range in the 3-7.5mg/kg dose group was 8.2-11.5 days.
It can be concluded that IMM27M monotherapy in general was well-tolerated at the dose levels evaluated and preliminarily single agent activity was seen in patients with heavily pretreated advanced solid tumors. The dose escalation study of IMM27M in advanced solid tumors is ongoing.
How to Easily View the Clinical Results Using Synapse Database?
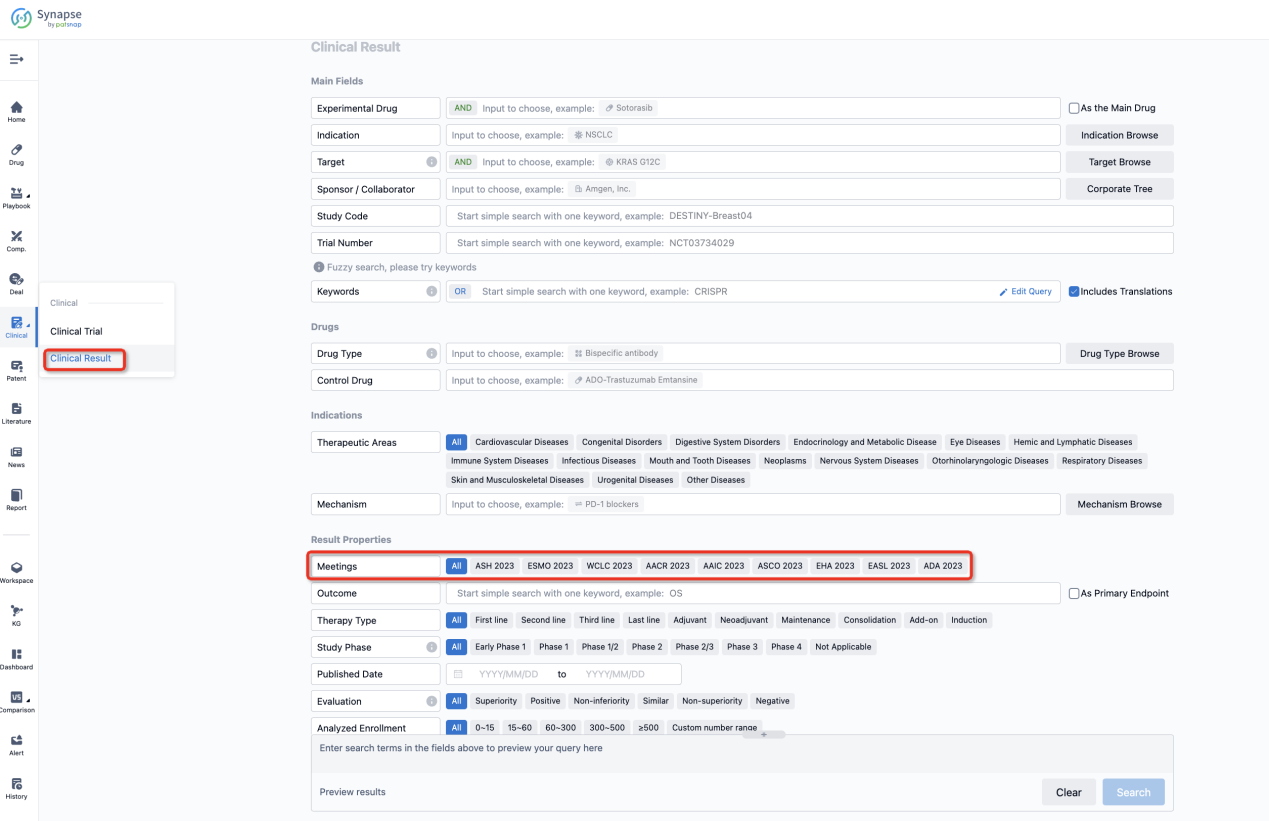
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
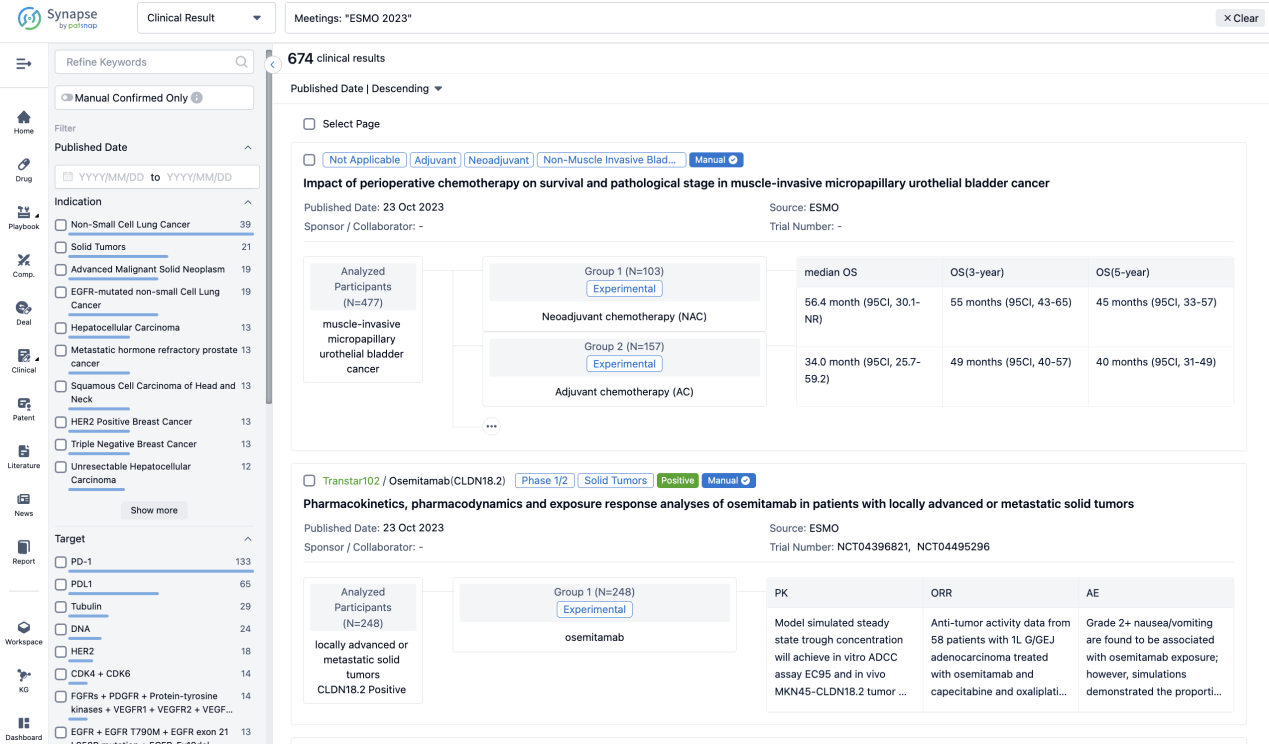
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

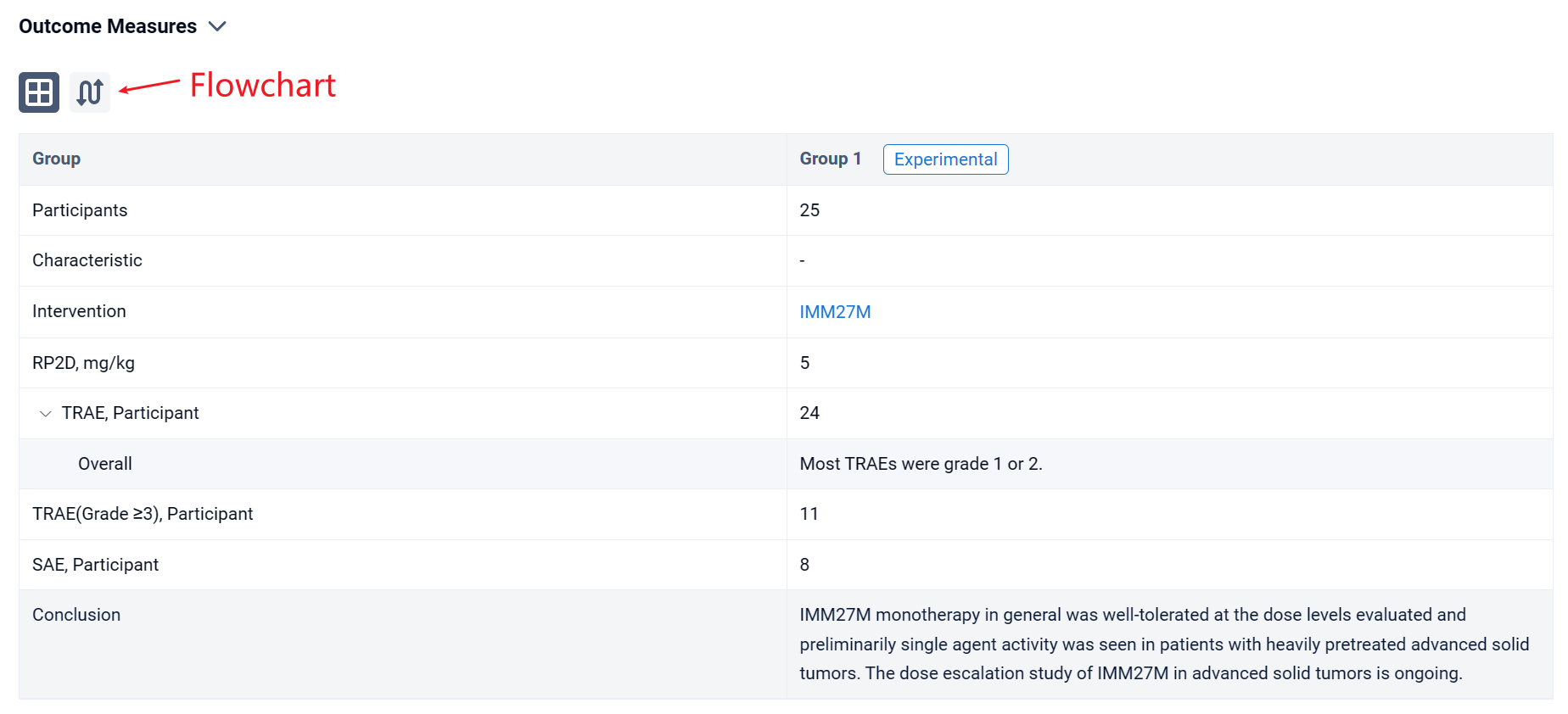
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
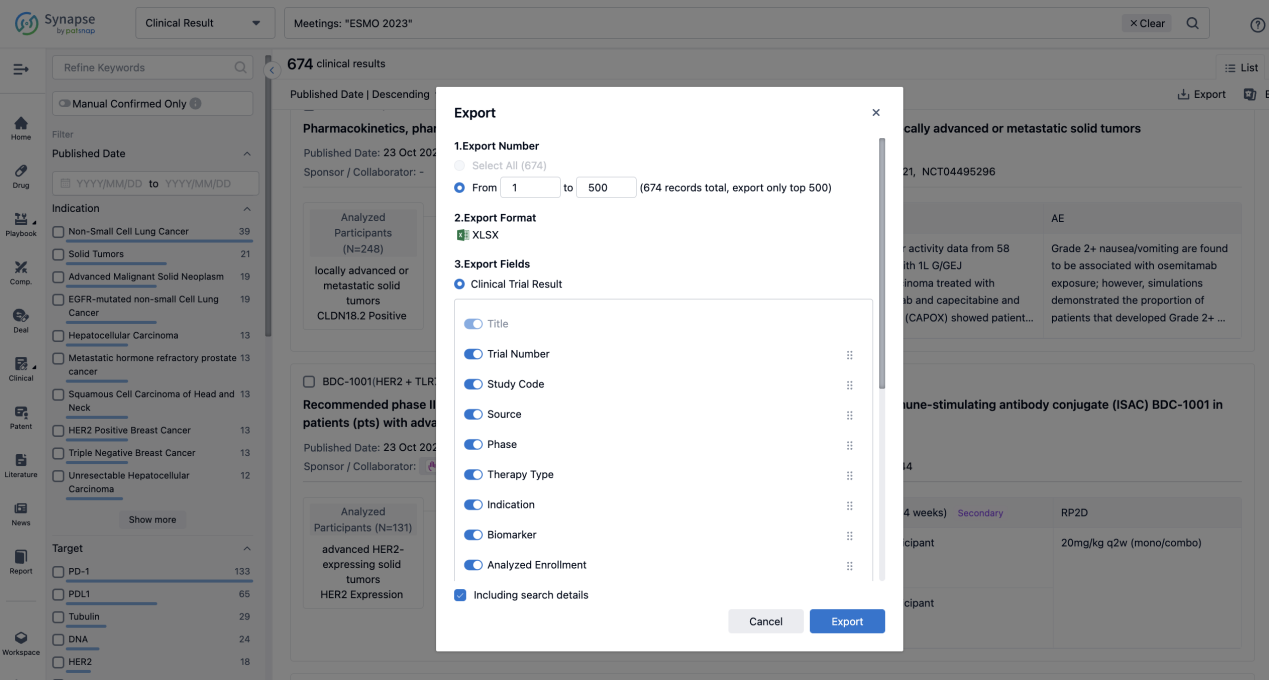
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!