An analysis of Mitomycin's R&D progress and its clinical results presented at the 2024 ASCO_GU Annual Meeting
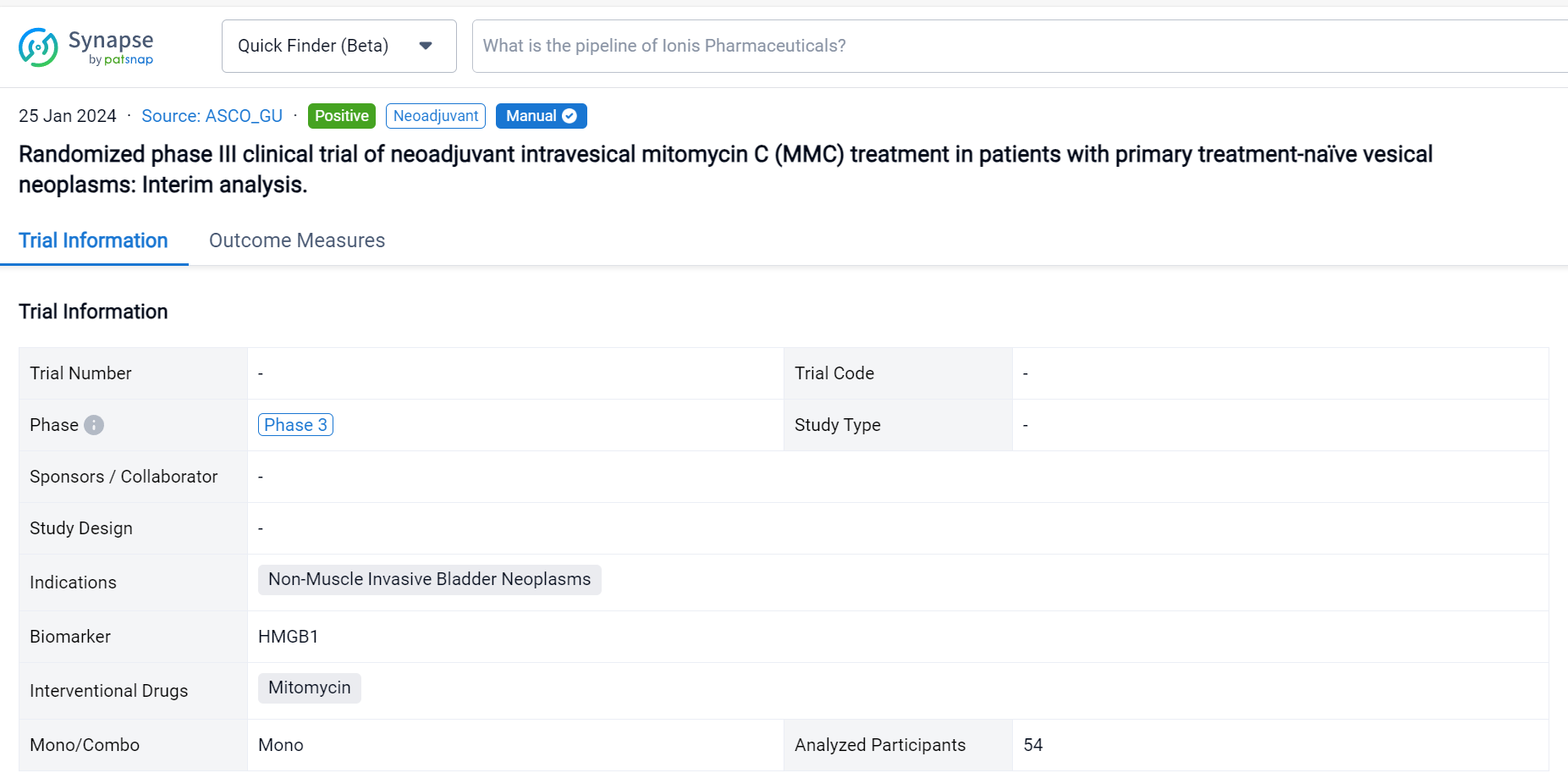
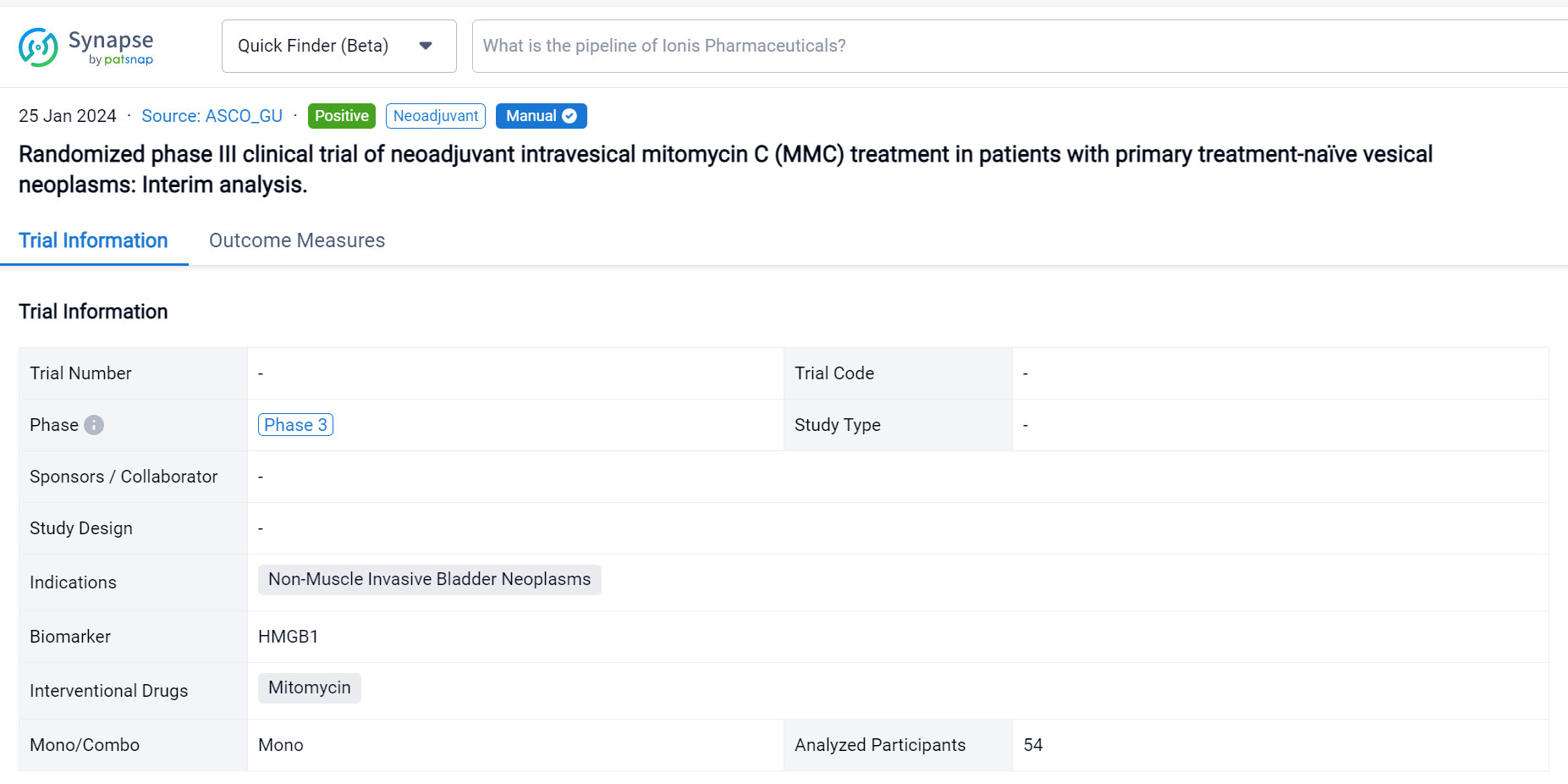
On 25 Jan 2024, the randomized phase III clinical trial of neoadjuvant intravesical mitomycin C (MMC) treatment in patients with primary treatment-naïve vesical neoplasms was reported in 2024 ASCO_GU.
Mitomycin's R&D Progress
Mitomycin is a small molecule drug that falls under the therapeutic areas of neoplasms, immune system diseases, digestive system disorders, endocrinology and metabolic disease, eye diseases, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases.
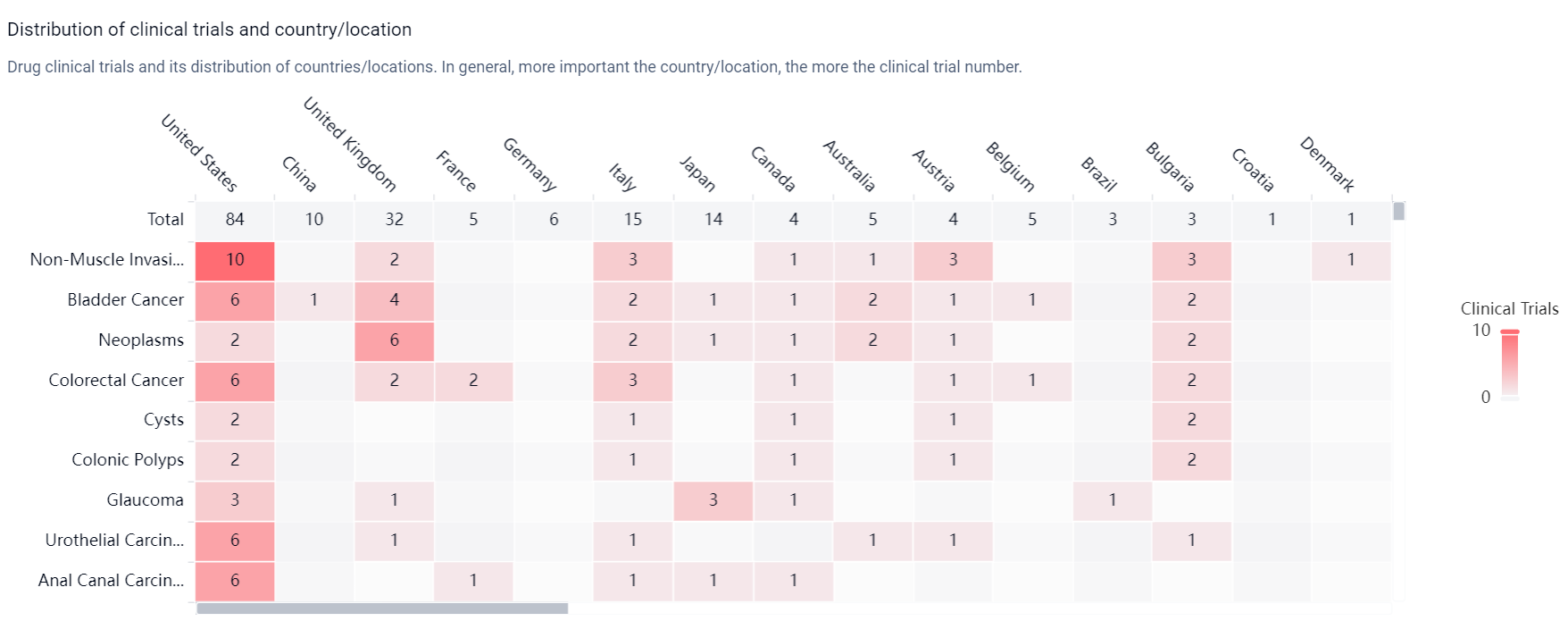
According to the Patsnap Synapse, Mitomycin was first approved in Japan in September 1963 and is developed by Kyowa Kirin Co., Ltd. It has received approvals in multiple countries and is currently in the highest phase of development, which is approved. And the clinical trial distributions for Mitomycin are primarily in the United States, China and United Kingdom. The key indication is Non-Muscle Invasive Bladder Neoplasms.
Detailed Clinical Result of Mitomycin
This prospective phase III randomized clinical trial (EUCTR2021-003751-42) was aimed to evaluate the safety, tolerability, and efficacy of neoadjuvant MMC in patients (pts) with non-muscle invasive bladder cancer (NMIBC).
In this study, pts with primary clinical diagnosis of urinary bladder cancer or secondary recurrent untreated bladder cancer since May 2022 to September 2023 (EudraCT 2021-003751-42_studio ICH-013-) are randomized 1:1 to neoadjuvant MMC arm (neoA) or standard arm (StA). In the 2 weeks before scheduled TUR (day 0) neoA pts receive intravesical instillations of MMC (40 mg/40 ml saline) at day -14 and -7 and cystoscopy with a cold cup biopsy at -14. After TUR, clinical choices both in the two groups, depend on histological evaluation of the tumour and EAU guidelines. Midstream and catheter urines are collected before instillations, at day 0 and after 3 months to respectively measure the level of specific biomarker (i.e., HMGB1) and to identify the microbiota. The expression of a mitomarker is evaluated on tissue samples. The primary endpoint is to assess safety, tolerability, and efficacy of neoadjuvant MMC in reducing the recurrence rate of BC calculated as the proportion of pts who achieve a complete response (no evidence of BC after 3, 6, 12 and 24 mo). The secondary endpoint will be the analysis of the rate of grade and stage progression to MIBC in case of recurrence and the correlation with biomarkers.
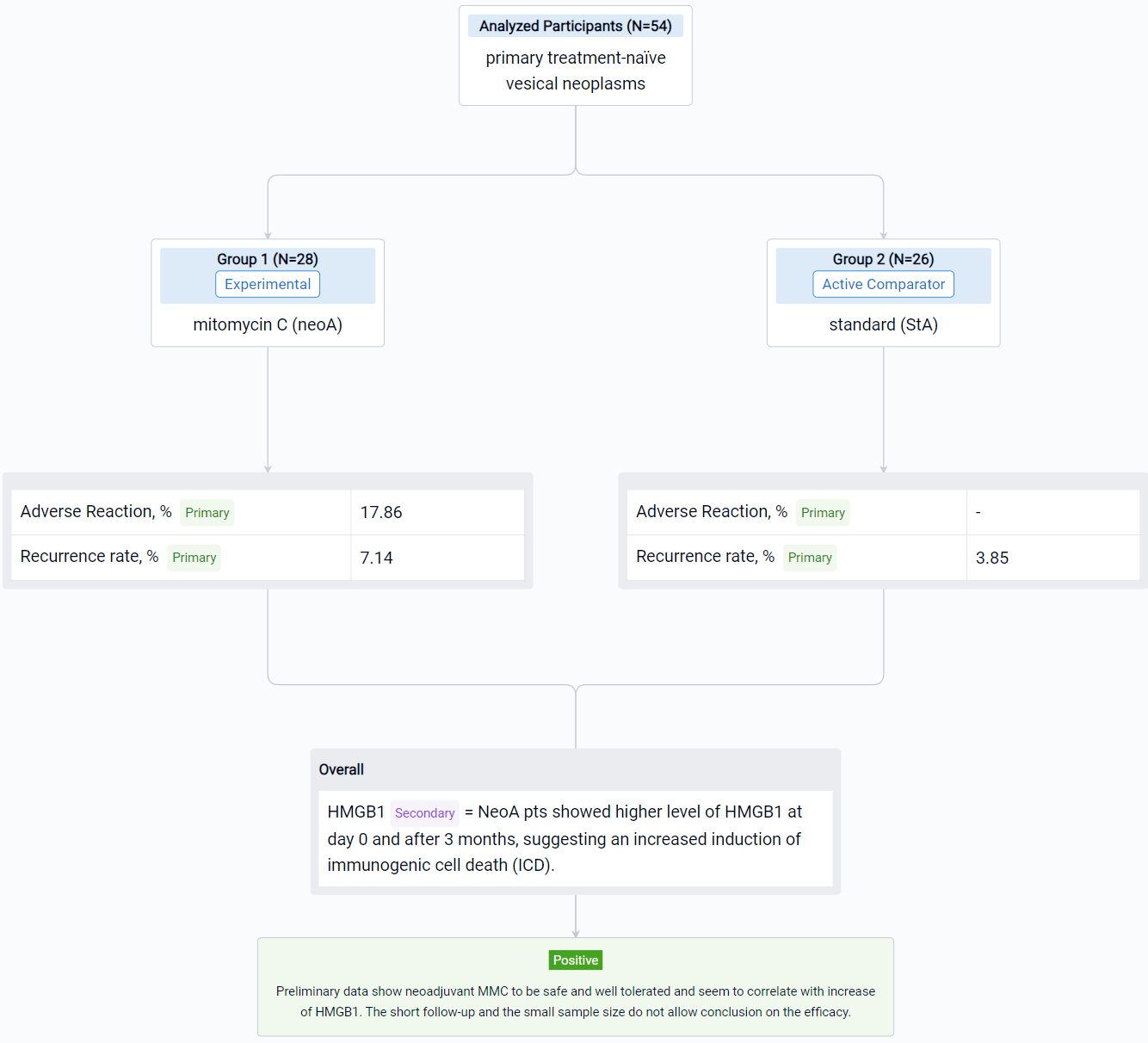
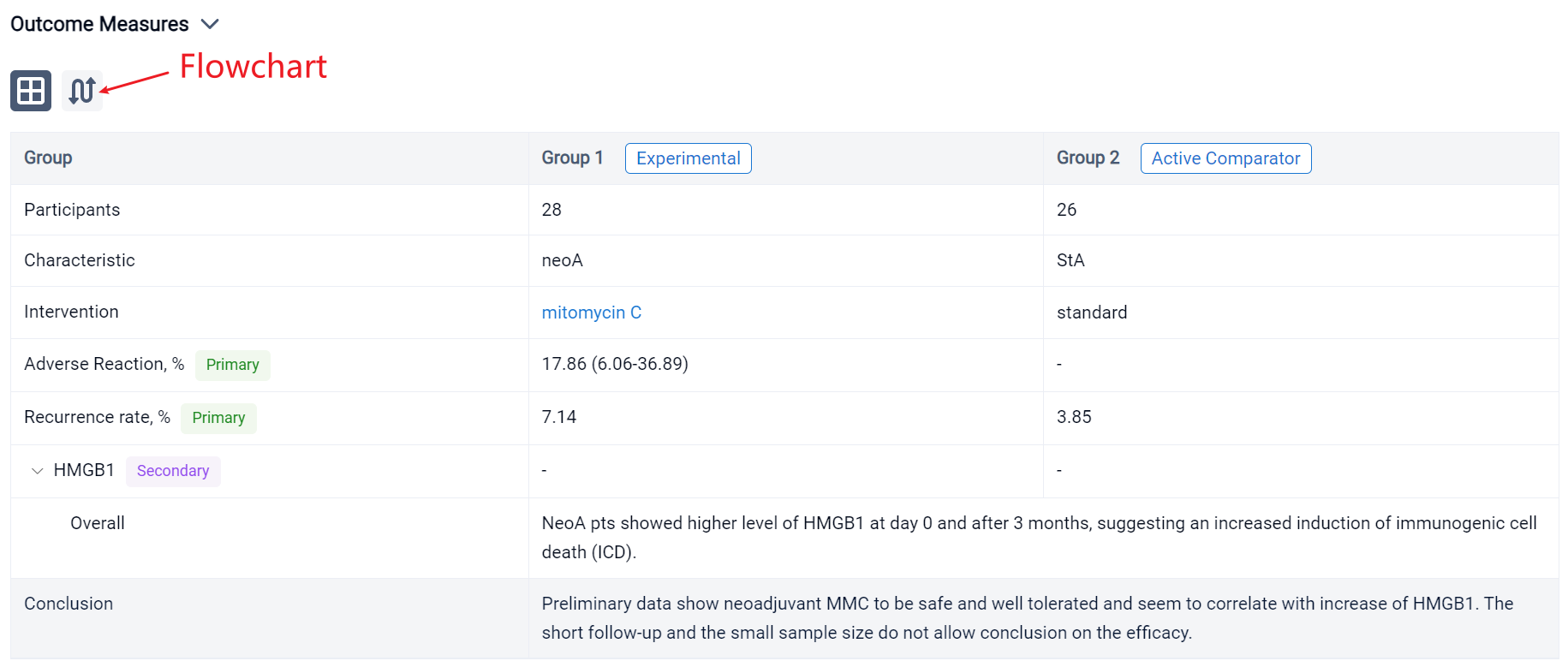
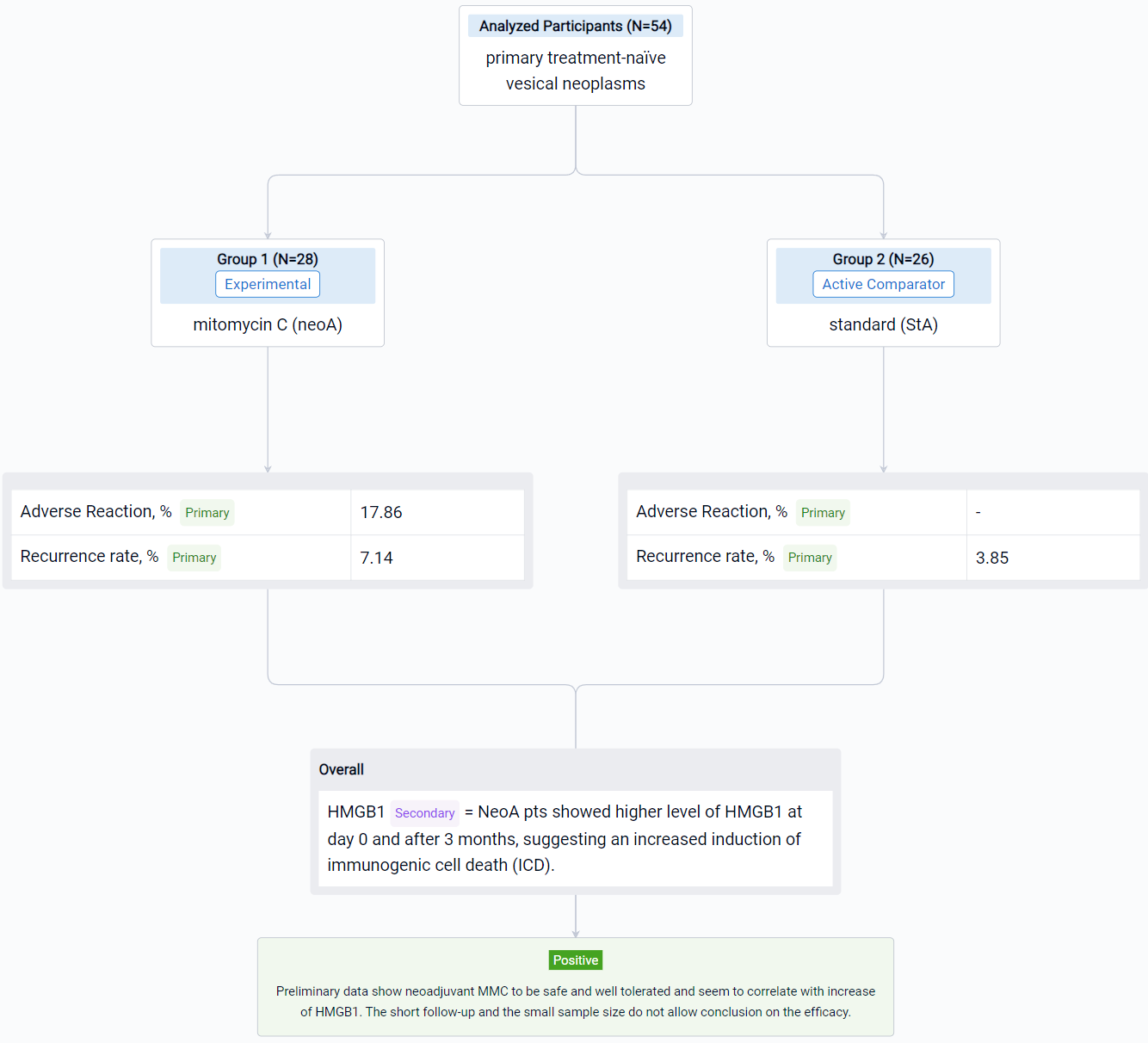
The result showed that respectively in StA and neoA mean age at TUR was 66.5 (±9.9) and 65.8 (±9.9), males were 23 (88.46%) and 24 (85.71%). No statistically significant difference was observed between groups. 5 neoA pts [17.86% (95%CI 6.06-36.89)] after instillations reported 4 different adverse reactions, grade 1 of Clavien Dindo classification. NeoA pts showed higher level of HMGB1 at day 0 and after 3 months, suggesting an increased induction of immunogenic cell death (ICD). Shotgun metagenomics on microbial DNA from catheter urine is still ongoing. In field recurrence has been observed in 1 (3.85%) StA and 2 (7.14%) neoA pts.
It can be concluded that neoadjuvant MMC to be safe and well tolerated and seem to correlate with increase of HMGB1. The short follow-up and the small sample size do not allow conclusion on the efficacy.
How to Easily View the Clinical Results Using Synapse Database?
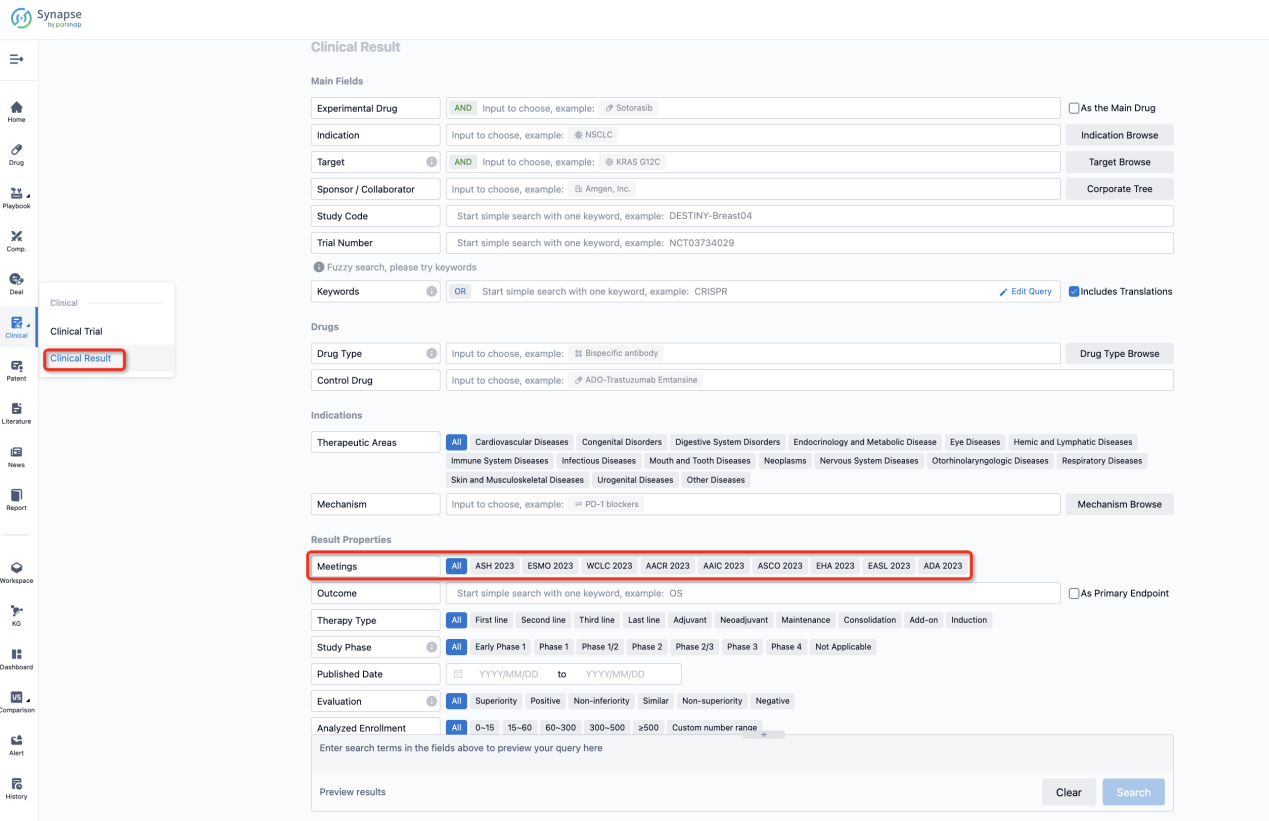
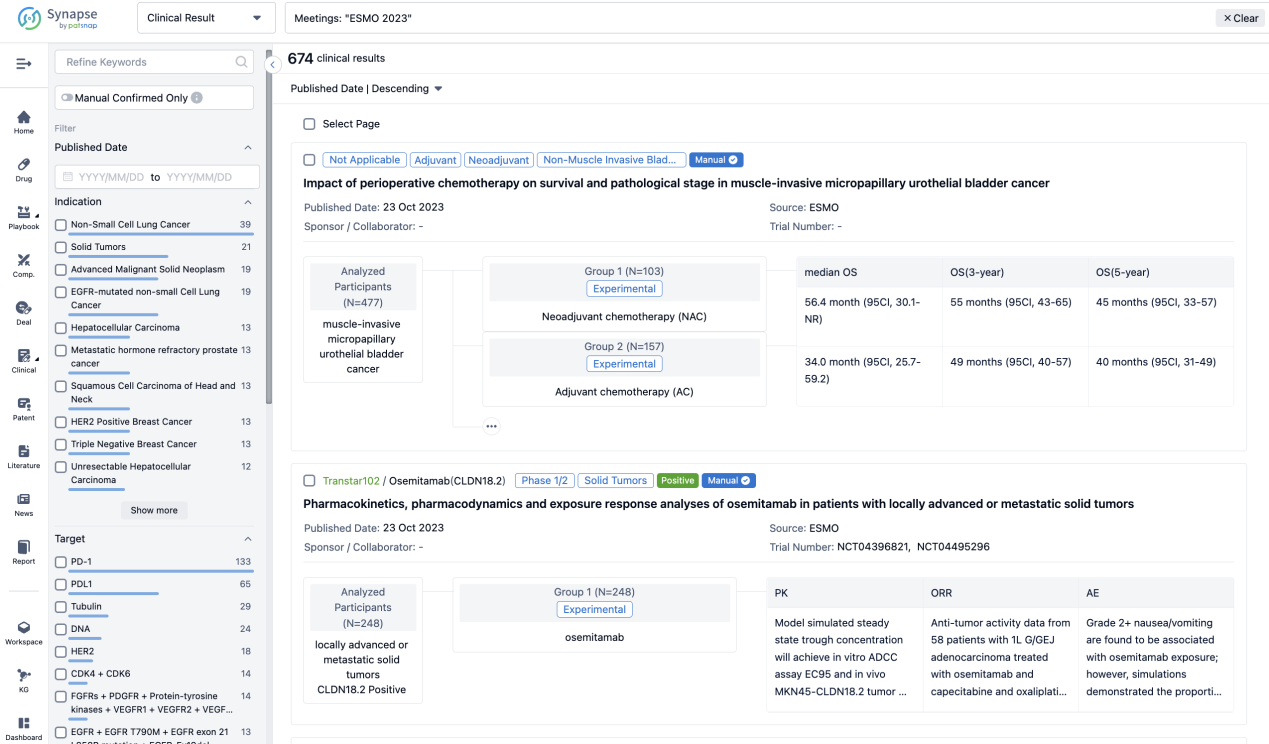
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
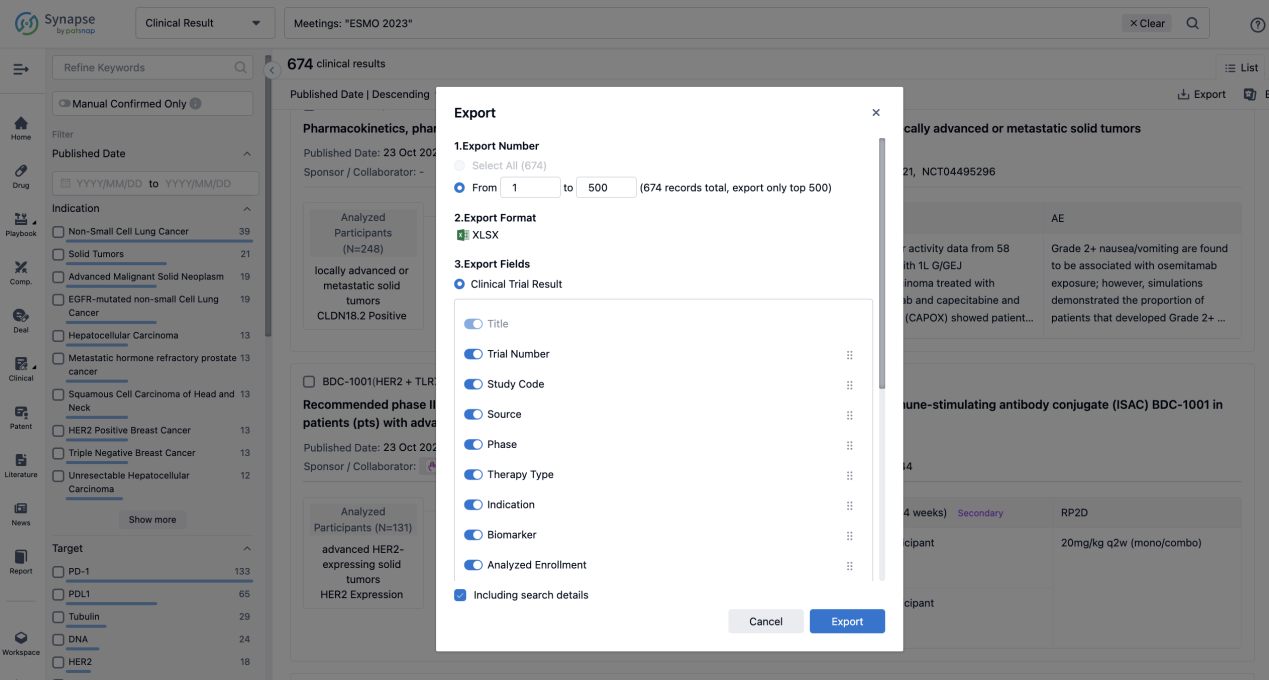
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!