Innovent's Phase 3 RESTORE-1 Study of IBI311 for Thyroid Eye Disease Hits Key Goal, Eyes NMPA Filing
Innovent Biologics, Inc. has reported successful outcomes for the key goal in the third-phase trial of IBI311, which is a synthesized antibody targeting the IGF-1R, for the patient population in China suffering from Thyroid Eye Disease (TED). The company is preparing to file for approval of IBI311 as a therapeutic option for TED with the National Drug Administration's Center for Drug Evaluation.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
RESTORE-1, a multi-site, controlled, blinded, and randomized study in Phase 2/3, assesses the performance and safety of the therapeutic agent IBI311 in participants diagnosed with Thyroid Eye Disease (TED). By the conclusive 24th week of the RESTORE-1's Phase 3 segment, the chief objective to gauge the proportion of subjects showing a substantial proptosis reduction in the affected eye was achieved.
Moreover, pivotal secondary outcomes within the investigation, which included the combined rate of response, the portion of individuals exhibiting a Clinical Activity Score (CAS) that was reduced to 0 or 1, and the average reduction in proptosis of the affected eye when compared to initial measurements, were also met, demonstrating that IBI311 had a significant effect relative to the given placebo.
With respect to safety, IBI311's profile was positively received, maintaining a record of no severe adverse events. The Phase 3 component's data on efficacy and safety mirrored the findings from Phase 2. Comprehensive findings from the RESTORE-1 will be disseminated at future scientific symposiums and through peer-reviewed medical publications.
As an inflammatory condition of the eye's orbit, Thyroid Eye Disease (TED) has an estimated annual occurrence of 16 cases per 100,000 females and 2.9 per 100,000 males. The projected frequency for clinically significant TED lies between 0.1% and 0.3%. Although there are no specifically designated medications for TED treatment authorized in China at this time, various authoritative clinical protocols advise the application of Insulin-like Growth Factor-I Receptor (IGF-IR) targeted antibodies for patients with TED. Specifically, for individuals experiencing marked proptosis, the IGF-IR targeted antibody is suggested as a principal treatment option.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
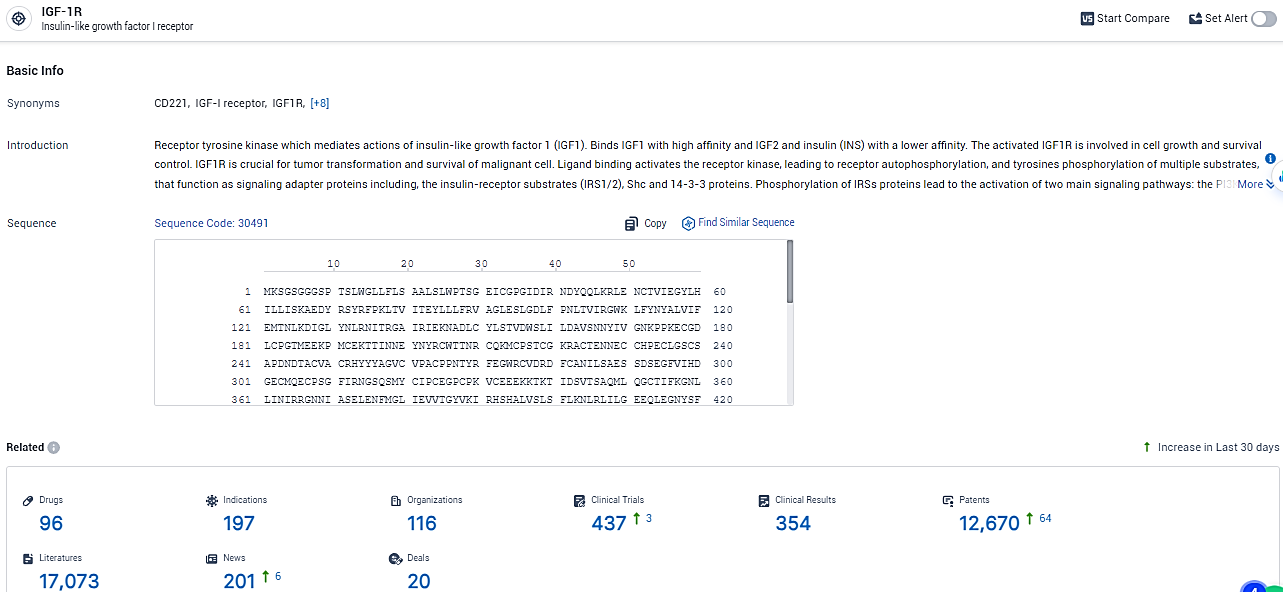
According to the data provided by the Synapse Database, As of February 23, 2024, there are 96 investigational drugs for the IGF-1R target, including 197 indications,116 R&D institutions involved, with related clinical trials reaching 437, and as many as 12670 patents.
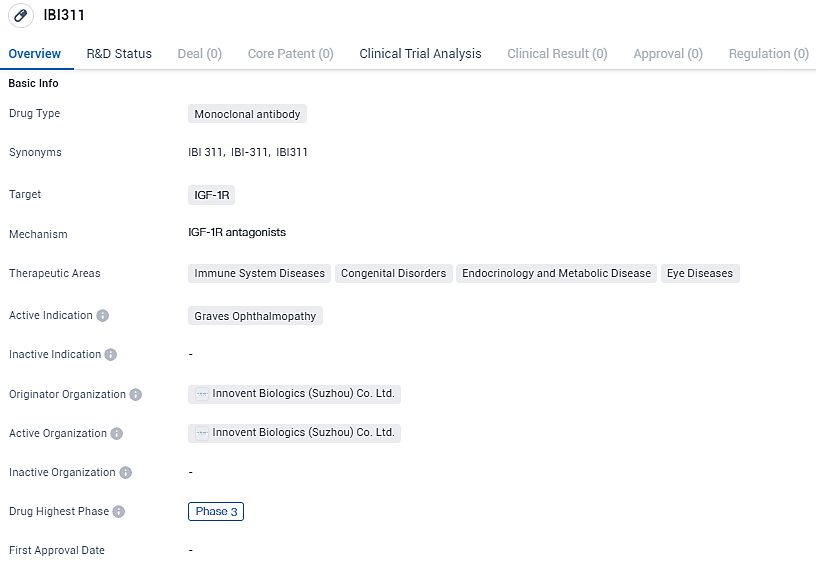
IBI311 targets the IGF-1R and is being developed for the treatment of Graves Ophthalmopathy. Currently in Phase 3 of clinical development, IBI311 has the potential to address the unmet medical needs of patients with this condition.