An In-depth Analysis of Celastrol's R&D Progress and Mechanism of Action on Drug Target
Celastrol's R&D Progress
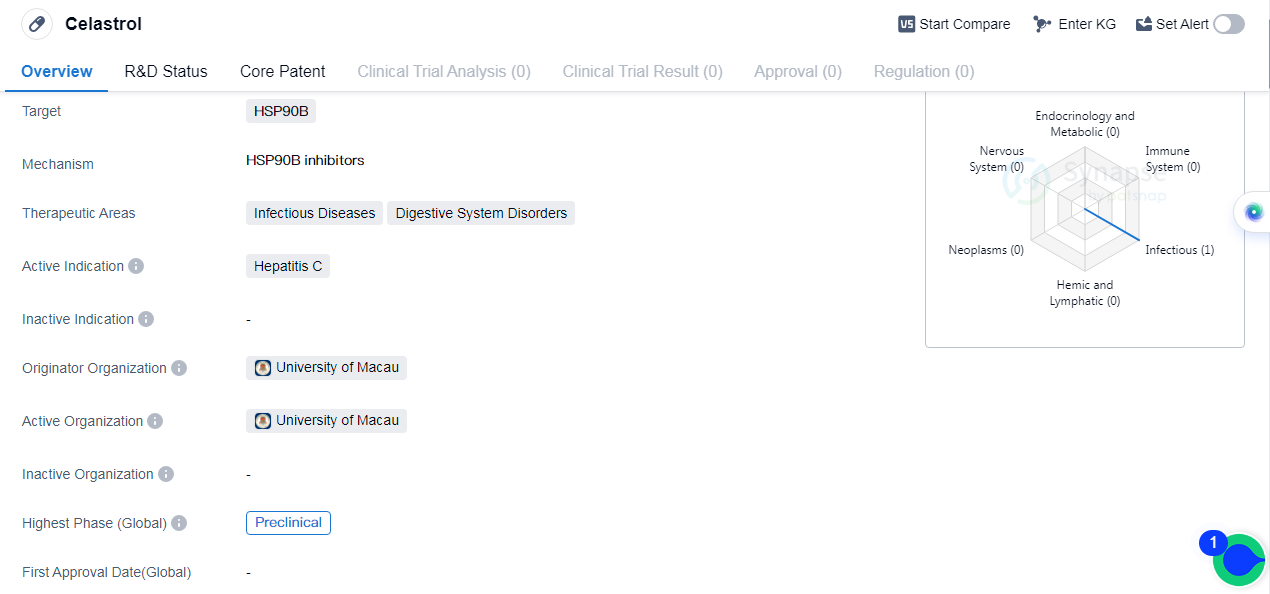
Celastrol is a small molecule drug that developed to target HSP90B, which is a protein involved in various cellular processes. The drug is primarily investigated for its potential therapeutic applications in infectious diseases and digestive system disorders. Specifically, it is being explored as a treatment for hepatitis C, a viral infection that affects the liver.
The drug is currently in the preclinical stage of development, which means that it is being tested in laboratory and animal studies to evaluate its safety and efficacy. The highest phase of development for Celastrol is preclinical globally.
The originator organization of Celastrol is the University of Macau. It is worth noting that the University of Macau is a reputable academic institution known for its research and development activities in various fields, including biomedicine.
Celastrol's targeting of HSP90B suggests that it may have potential therapeutic benefits in diseases and disorders where this protein is implicated. HSP90B is known to play a role in various cellular processes, including protein folding and stabilization, making it an attractive target for drug development.
The therapeutic areas of infectious diseases and digestive system disorders are broad categories that encompass a wide range of conditions. Celastrol's potential application in hepatitis C indicates that it may have antiviral properties or could help alleviate the symptoms associated with this particular infection.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Celastrol: HSP90B inhibitors
HSP90B inhibitors are a type of drugs that target and inhibit the activity of heat shock protein 90 beta (HSP90B), which is a molecular chaperone protein that plays a crucial role in maintaining the stability and function of various client proteins involved in cell signaling, growth, and survival. By inhibiting HSP90B, these inhibitors disrupt the chaperone function of HSP90B and lead to the degradation of client proteins, ultimately affecting cellular processes.
From a biomedical perspective, HSP90B inhibitors have gained significant attention in cancer research. Many client proteins of HSP90B are involved in promoting the growth and survival of cancer cells. By inhibiting HSP90B, these inhibitors can potentially block the activity of these client proteins, leading to the suppression of cancer cell growth and proliferation. Therefore, HSP90B inhibitors are being investigated as potential anticancer agents and are being studied in preclinical and clinical trials.
It is important to note that HSP90B inhibitors may have off-target effects and can affect the function of other proteins in addition to HSP90B. Therefore, extensive research is being conducted to understand the specificity and selectivity of these inhibitors to minimize potential side effects and optimize their therapeutic potential.
Drug Target R&D Trends for Celastrol
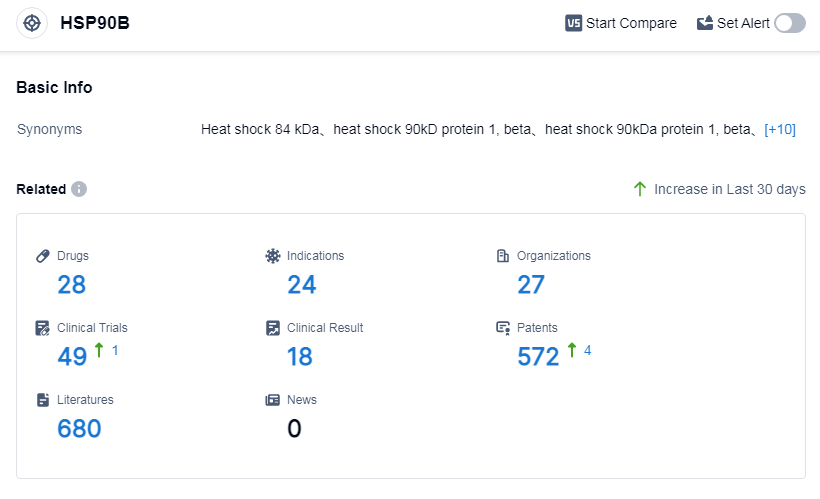
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 28 HSP90B drugs worldwide, from 27 organizations, covering 24 indications, and conducting 49 clinical trials.
Approved drugs for indications like Gastrointestinal Stromal Tumors highlight the therapeutic potential of targeting HSP90B. Small molecule drugs and monoclonal antibodies are the most rapidly progressing drug types, indicating intense competition and innovation. China is at the forefront of development, and show significant progress. Overall, the analysis suggests a promising future for the development of drugs targeting HSP90B.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Celastrol is a small molecule drug that developed by the University of Macau, with focus on HSP90B. Its potential therapeutic applications lie in the treatment of infectious diseases and digestive system disorders, particularly hepatitis C. While the drug is still in the preclinical stage of development, further research and clinical trials will be necessary to determine its safety and efficacy.