Istradefylline: Detailed Review of its Transformative R&D Success
Istradefylline's R&D Progress
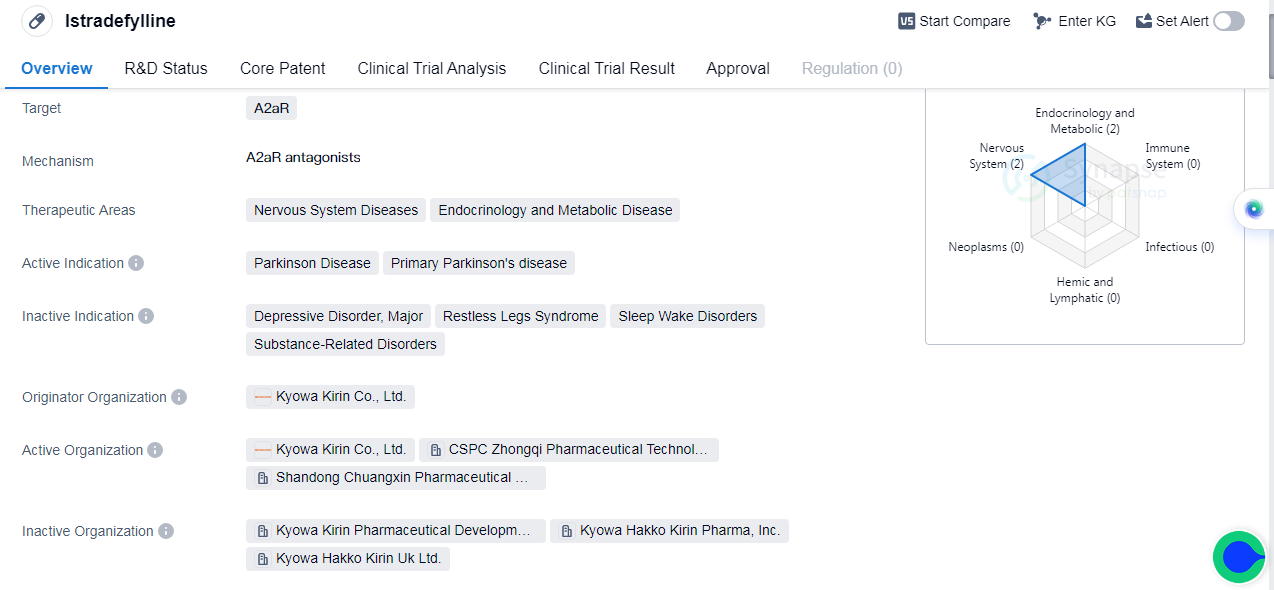
Istradefylline is a small molecule drug that targets the A2aR receptor. It is primarily used in the treatment of Parkinson's disease, specifically primary Parkinson's disease. The drug is developed by Kyowa Kirin Co., Ltd., a pharmaceutical company based in Japan.
In terms of therapeutic areas, Istradefylline is mainly focused on addressing nervous system diseases and endocrinology and metabolic diseases. It has achieved approval in the global market, this means that the drug has successfully completed all necessary clinical trials and has been granted regulatory approval for treatment of Parkinson's disease. The first approval for Istradefylline was granted in Japan in March 2013.
In China, Istradefylline is currently in phase 3 of clinical development. This indicates that the drug is being tested in a larger population to further evaluate its safety and efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Istradefylline: A2aR antagonists
A2aR antagonists refer to a class of drugs or compounds that specifically target and block the adenosine A2a receptor (A2aR). Adenosine A2a receptors are a type of G protein-coupled receptor found on the surface of cells. They are primarily expressed in the central nervous system, immune cells, and various other tissues.
From a biomedical perspective, A2aR antagonists have been extensively studied for their potential therapeutic applications, particularly in the field of immunology and neurology. Adenosine A2a receptors play a crucial role in regulating immune responses and inflammation. By inhibiting or blocking these receptors, A2aR antagonists can modulate immune cell function and reduce inflammation.
In the context of biomedicine, A2aR antagonists have shown promise as potential treatments for autoimmune diseases and neurodegenerative disorders. By blocking A2a receptors, these antagonists can potentially suppress excessive immune responses and alleviate inflammation associated with these conditions.
Furthermore, A2aR antagonists have also been investigated for their potential role in cancer therapy. Adenosine signaling through A2a receptors can promote tumor growth, immune evasion, and resistance to chemotherapy. By blocking A2a receptors, these antagonists may enhance anti-tumor immune responses and increase the effectiveness of cancer treatments.
Overall, A2aR antagonists represent a class of drugs that target and inhibit adenosine A2a receptors, offering potential therapeutic benefits in the fields of immunology, neurology, and oncology.
Drug Target R&D Trends for Istradefylline
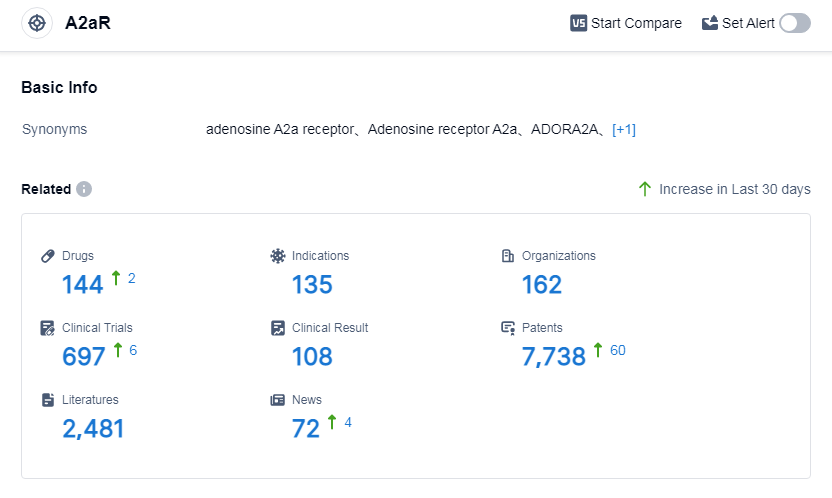
According to Patsnap Synapse, as of 5 Sep 2023, there are a total of 144 A2aR drugs worldwide, from 162 organizations, covering 135 indications, and conducting 697 clinical trials.
The analysis of the current competitive landscape of target A2aR reveals that there are several companies actively involved in the research and development of drugs targeting this receptor. Nichi-Iko Pharmaceutical Co., Ltd., Kirin Holdings Co., Ltd., AbbVie, Inc., Otsuka Holdings Co., Ltd., and Huaxia Xinhe (Shanghai) Life Science Co., Ltd. are some of the companies with significant R&D progress in this area. Small molecule drugs are the most rapidly progressing drug type under this target, with intense competition around innovative drugs. China is the leading country in terms of drug development under this target, with a large number of approved drugs and ongoing preclinical research. Overall, the target A2aR presents a competitive landscape with multiple companies, diverse indications, and a focus on small molecule drugs, particularly in China.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Istradefylline is a small molecule drug developed by Kyowa Kirin Co., Ltd. that targets the A2aR receptor. It has been approved globally for the treatment of primary Parkinson's disease and has been available in Japan since 2013. The drug is also undergoing phase 3 clinical trials in China, indicating potential future availability in that market.