Review of Alkermes Plc’s Drug Pipeline | R&D Status | Therapeutic Areas
Alkermes Plc is a biopharmaceutical company focused on central nervous system (CNS) disorders such as schizophrenia, depression, addiction, and multiple sclerosis. The company was founded in 1987 by Michael Wall. In September 2011, Alkermes, Inc. merged with Elan Drug Technologies (EDT), the former drug formulation and manufacturing division of Élan Corporation, plc. Headquartered in Dublin, Ireland, the company has R&D centers in Waltham, Massachusetts, and manufacturing facilities in Athlone, Ireland and Wilmington, Ohio. Alkermes plc is a biopharmaceutical company dedicated to the research, development and commercialization of medicines to meet the unmet medical needs of patients in various therapeutic areas in the United States, Ireland and internationally. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Alkermes Plc.
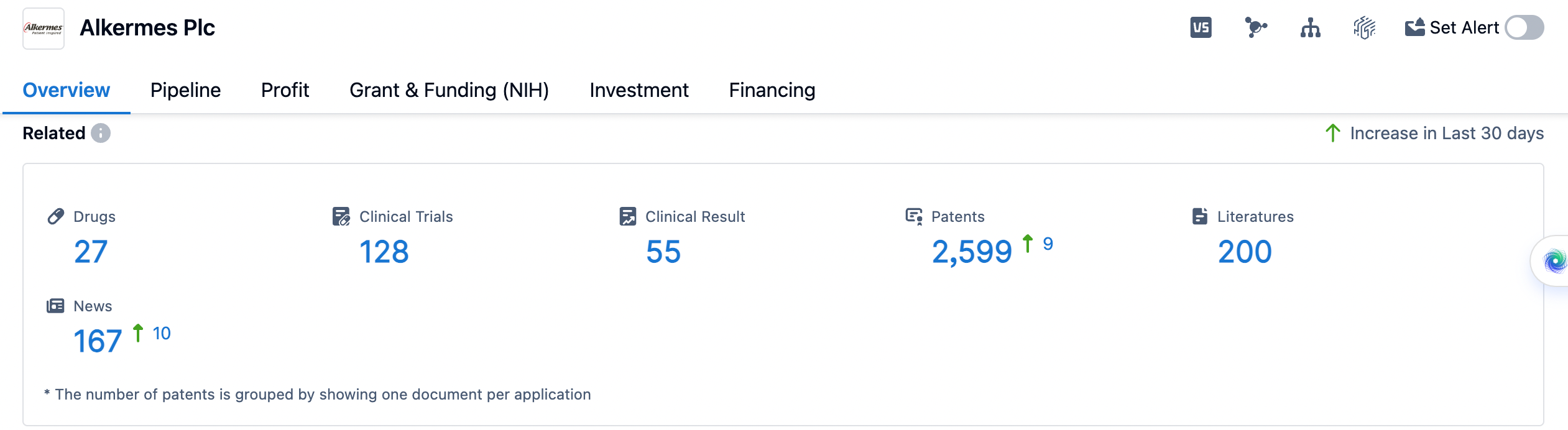
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Alkermes Plc.
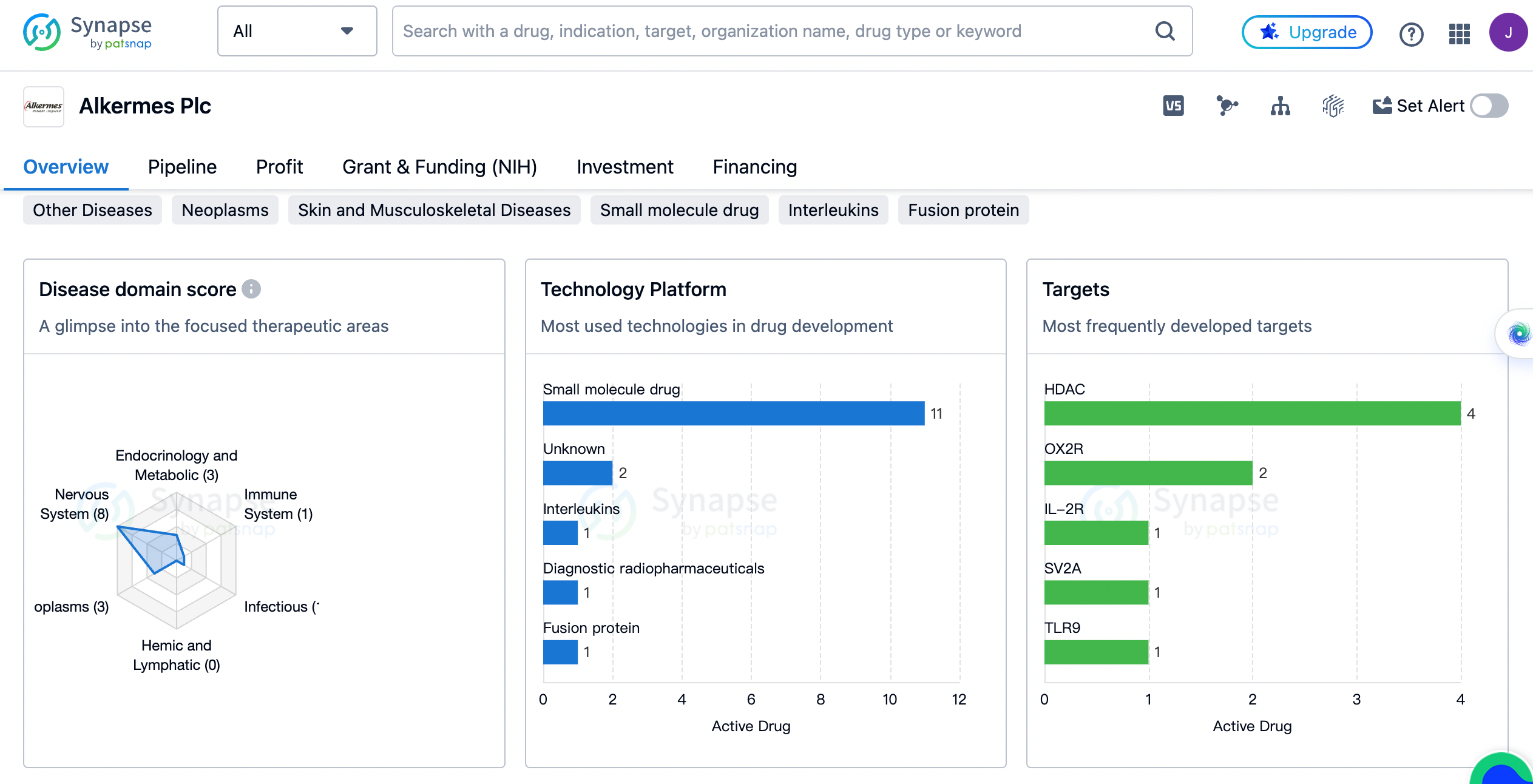
Alkermes Plc has developed drugs for a wide range of therapeutic areas. The highest number of drugs, 8 in total, are focused on Nervous System Diseases. This indicates that Alkermes Plc has a strong emphasis on developing treatments for neurological disorders. Other Diseases and Neoplasms follow closely with 7 and 3 drugs respectively. This suggests that Alkermes Plc also invests in developing drugs for various other diseases, including cancer. Respiratory Diseases, Endocrinology and Metabolic Disease, and Skin and Musculoskeletal Diseases each have 3 drugs developed by Alkermes Plc. The remaining therapeutic areas, including Digestive System Disorders, Immune System Diseases, Infectious Diseases, Urogenital Diseases, and Congenital Disorders, have 1 or 2 drugs developed by the organization.
The most frequently developed targets by Alkermes Plc.
HDAC, which stands for Histone Deacetylase, is the most targeted with 4 drugs developed. This indicates that Alkermes Plc is actively involved in researching and developing drugs that target HDAC. OX2R, IL-2R, SV2A, TLR9, and Nrf2 are some of the other targets that Alkermes Plc has focused on, with each having 1 or 2 drugs developed. It is worth noting that some drugs target multiple receptors, such as Opioid receptors + μ opioid receptor, 5-HT1A receptor + 5-HT2A receptor + D2 receptor, and 5-HT2 receptor + D1 receptor + D2 receptor + μ opioid receptor.
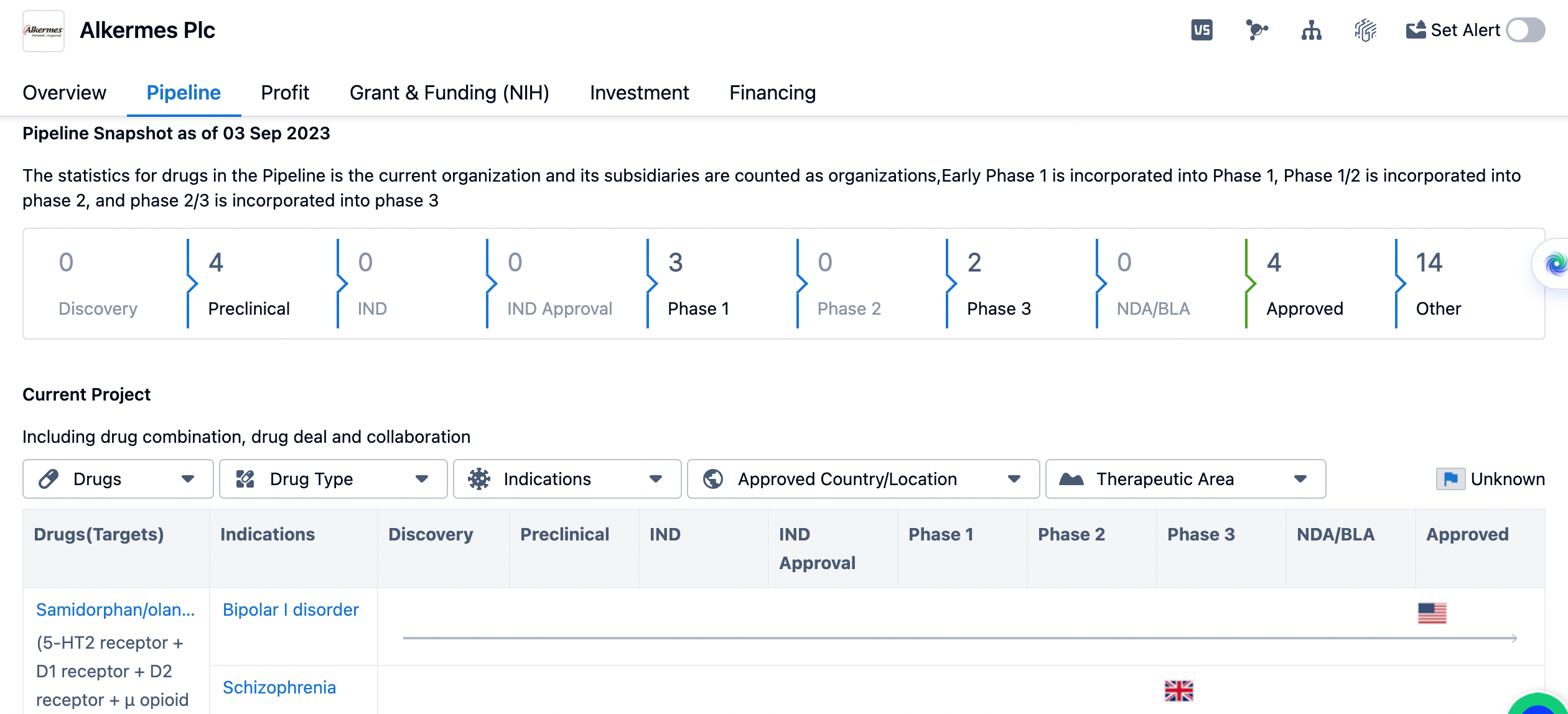
The pipeline of Alkermes Plc until September, 2023.
The pipeline is divided into different phases of drug development. Currently, Alkermes Plc has 4 drugs in the preclinical stage, indicating that they are actively researching and testing these drugs before moving on to the next phases. There are no drugs in the discovery stage. However, there are 3 drugs in Phase 1, indicating that they have progressed to the initial stages of clinical trials. Phase 3 has 2 drugs, indicating that they have advanced to the late stages of clinical trials. None of the drugs have reached the NDA/BLA (New Drug Application/Biologics License Application) stage, which is the final step before seeking regulatory approval. However, Alkermes Plc has 4 drugs that have been approved, indicating that they have successfully completed the drug development process for these medications. Additionally, there are 14 drugs listed under "Other," which could include drugs in various stages of development or those that do not fit into the traditional pipeline phases.
In summary, Alkermes Plc is a pharmaceutical organization based in Dublin, Ireland, specializing in the development of drugs for various therapeutic areas. They have a strong focus on Nervous System Diseases, with the highest number of drugs developed in this area. Alkermes Plc also invests in developing drugs for Other Diseases, Neoplasms, Respiratory Diseases, Endocrinology and Metabolic Disease, and Skin and Musculoskeletal Diseases. The organization targets a variety of receptors, with HDAC being the most frequently targeted. Alkermes Plc has a pipeline of drugs at different stages of development, with a significant number in the preclinical stage. They have drugs in Phase 1 and Phase 3, indicating progress in clinical trials. Alkermes Plc has successfully obtained approval for 4 drugs and has several others in various stages of development. Overall, Alkermes Plc demonstrates a commitment to advancing biomedicine and addressing unmet medical needs in multiple therapeutic areas.