ACE inhibitor -Overview of its R&D Progress | Competitive Landscape | Key Drug
ACE, or angiotensin-converting enzyme, plays a crucial role in the human body by regulating blood pressure and fluid balance. It converts angiotensin I, a hormone produced by the liver, into angiotensin II, a potent vasoconstrictor that narrows blood vessels. This process increases blood pressure and stimulates the release of aldosterone, a hormone that promotes sodium and water retention. ACE also breaks down bradykinin, a peptide that causes blood vessels to dilate and lowers blood pressure. Due to its involvement in these important physiological processes, ACE inhibitors are commonly used in the treatment of hypertension, heart failure, and other cardiovascular conditions.
ACE Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 2 Sep 2023, there are a total of 163 ACE drugs worldwide, from 159 organizations, covering 68 indications, and conducting 810 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.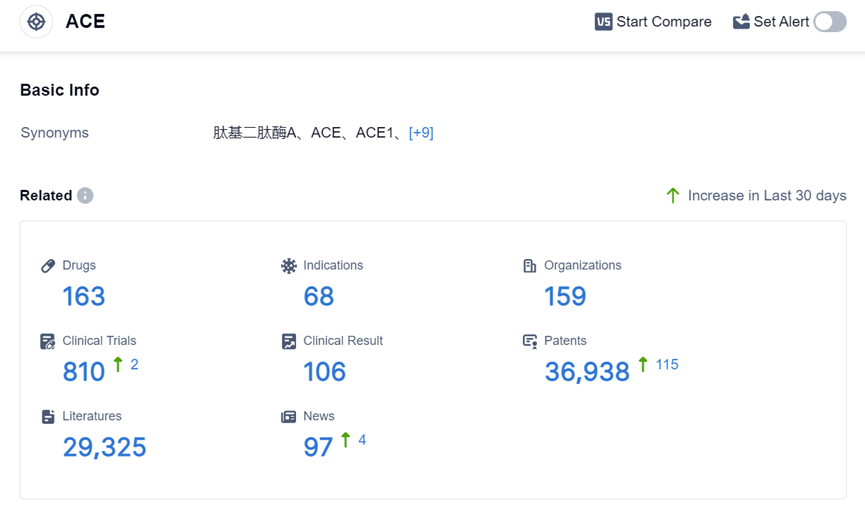
The analysis of the target ACE reveals a competitive landscape with companies like Les Laboratoires Servier SAS, CHIESI Farmaceutici SpA, and Viatris Inc. leading in R&D efforts. The focus of research is primarily on indications like hypertension, heart failure, and cardiovascular diseases. Small molecule drugs dominate the market, but there is also potential in other drug types like peptide drug conjugates and monoclonal antibodies.
The European Union, China, the United States, and Japan are the key regions driving the development of ACE-related drugs. China, in particular, has shown significant progress. Overall, the target ACE presents opportunities for further research and development, with potential for innovative drugs and intense competition in the market.
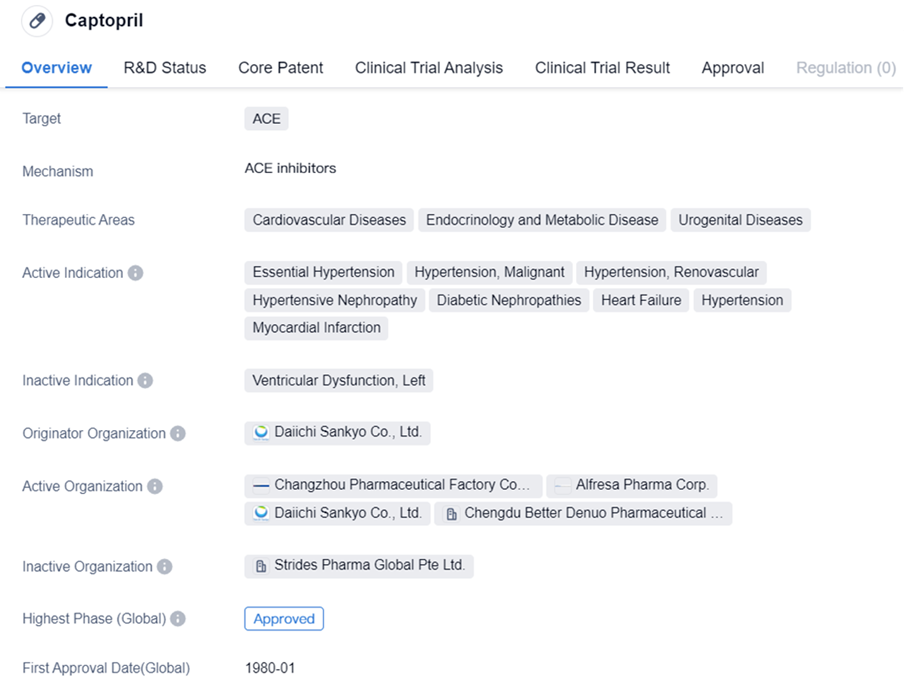
Key Drug: Captopril
Captopril is a small molecule drug that targets ACE and is used in the treatment of various cardiovascular, endocrinology, and urogenital diseases. It has been approved for multiple indications, including essential hypertension, hypertensive nephropathy, heart failure, and myocardial infarction. This wide range of indications highlights the versatility and effectiveness of Captopril in managing different conditions related to the cardiovascular system and metabolic disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Captopril was first approved in Japan in January 1980, making it a well-established drug with a long history of use. The drug has achieved the highest phase of approval both globally and in China, indicating its widespread acceptance and recognition by regulatory authorities. This suggests that Captopril has undergone rigorous testing and evaluation to ensure its safety and efficacy
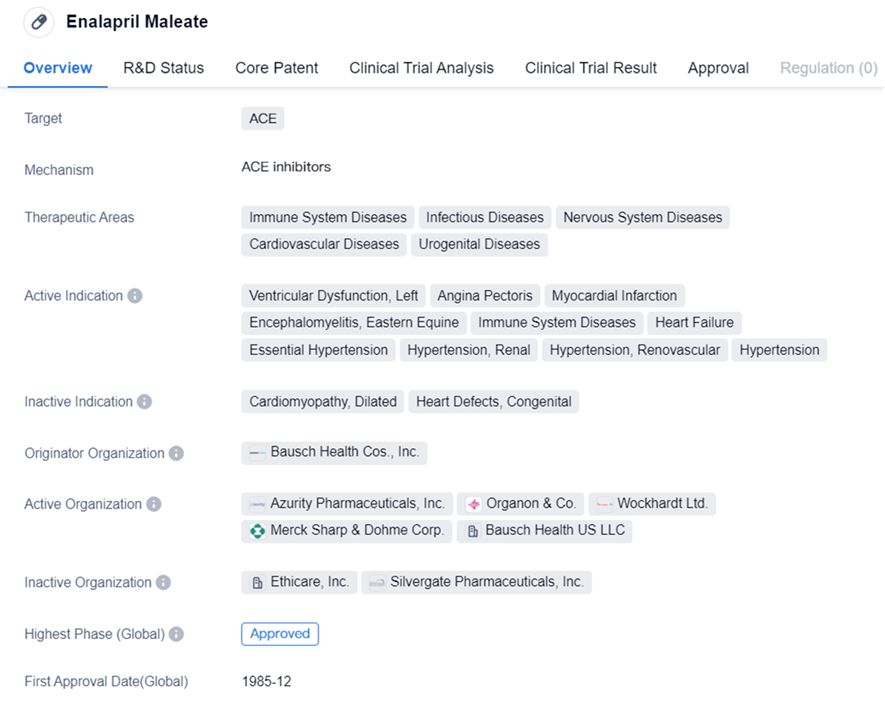
Enalapril Maleate
Enalapril Maleate is a small molecule drug that primarily targets ACE. It is used in the treatment of various therapeutic areas including immune system diseases, infectious diseases, nervous system diseases, cardiovascular diseases, and urogenital diseases. The drug has been approved for the treatment of ventricular dysfunction, left, angina pectoris, myocardial infarction, encephalomyelitis, eastern equine, immune system diseases, heart failure, essential hypertension, hypertension, renal, hypertension, renovascular, and hypertension.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Enalapril Maleate has a long history of use since its first approval in 1985. Its broad therapeutic areas and indications make it a versatile drug in the field of biomedicine. The drug's approval in both the global and Chinese markets further solidifies its position as a widely recognized and accepted treatment option. As a small molecule drug targeting ACE, Enalapril Maleate plays a crucial role in managing various diseases and conditions, particularly those related to the immune system, cardiovascular system, and nervous system.
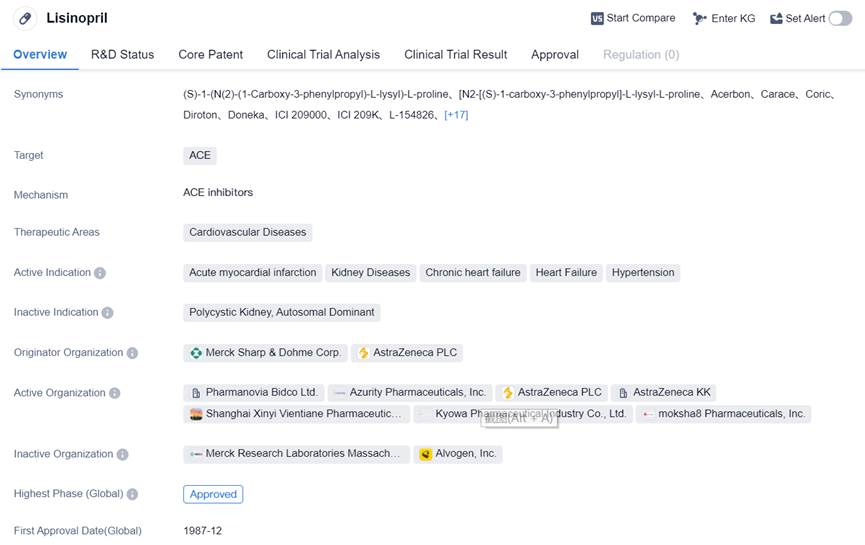
Lisinopril
Lisinopril is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It primarily targets ACE and is used to treat various conditions related to the heart and blood vessels. The drug has been approved for use in multiple indications, including acute myocardial infarction, kidney diseases, chronic heart failure, hypertension, and heart failure.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Lisinopril was first approved in the United States in December 1987 and has been developed by Merck Sharp & Dohme Corp. and AstraZeneca PLC. Lisinopril has reached the highest phase of development, indicating its safety and efficacy, and has received regulatory approval both globally and in China.