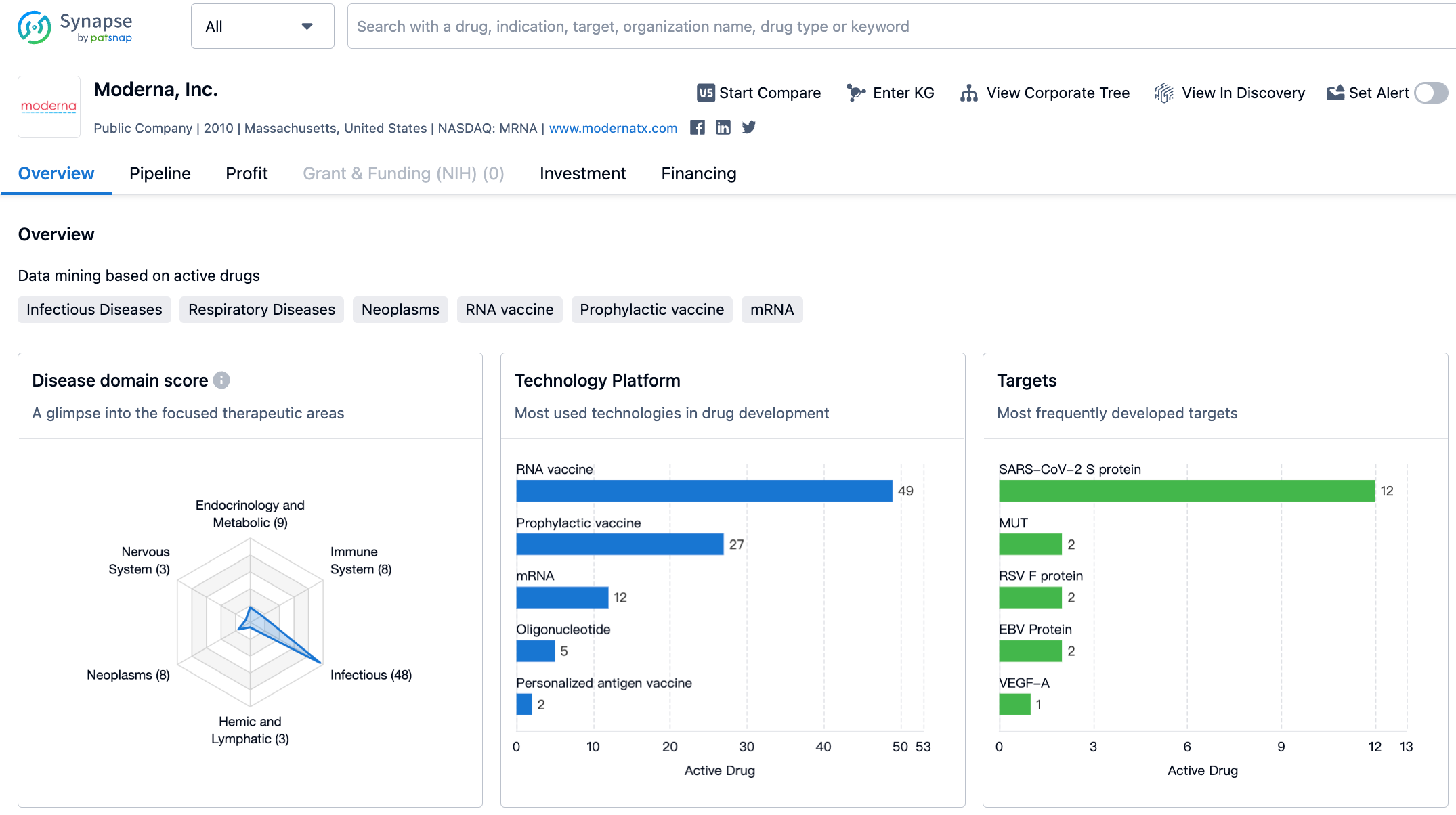
Analysis of Moderna's Cancer Vaccine Pipeline
The success of the COVID-19 vaccine not only promoted the development of mRNA technology, but also turned numerous pharmaceutical companies around overnight.
For instance, in the 2021 fiscal year, Moderna managed to reverse years of losses with just one commercial product, Spikevax. Its net profit soared to $12.2 billion, an increase of 1733.33% over the previous year. However, as the COVID-19 pandemic gradually winds down two years later, the question for Moderna is how should it seek to accelerate its development.
Transition to the field of oncology
One of Moderna's choices is to turn its focus to the oncology field.
Tumor vaccines, especially therapeutic ones, have always been coveted. The most recent prediction related to this occurred last October when the founder of BioNTech declared that its cancer vaccine is expected to hit the market before 2030.
In October 2022, Merck exercised its option to co-develop and commercialize mRNA-4157/V940 with Moderna for adjuvant treatment in high-risk melanoma patients. Based on the clinical data from KEYNOTE-94, in February, this vaccine was granted Breakthrough Therapy Designation (BTD) by FDA.
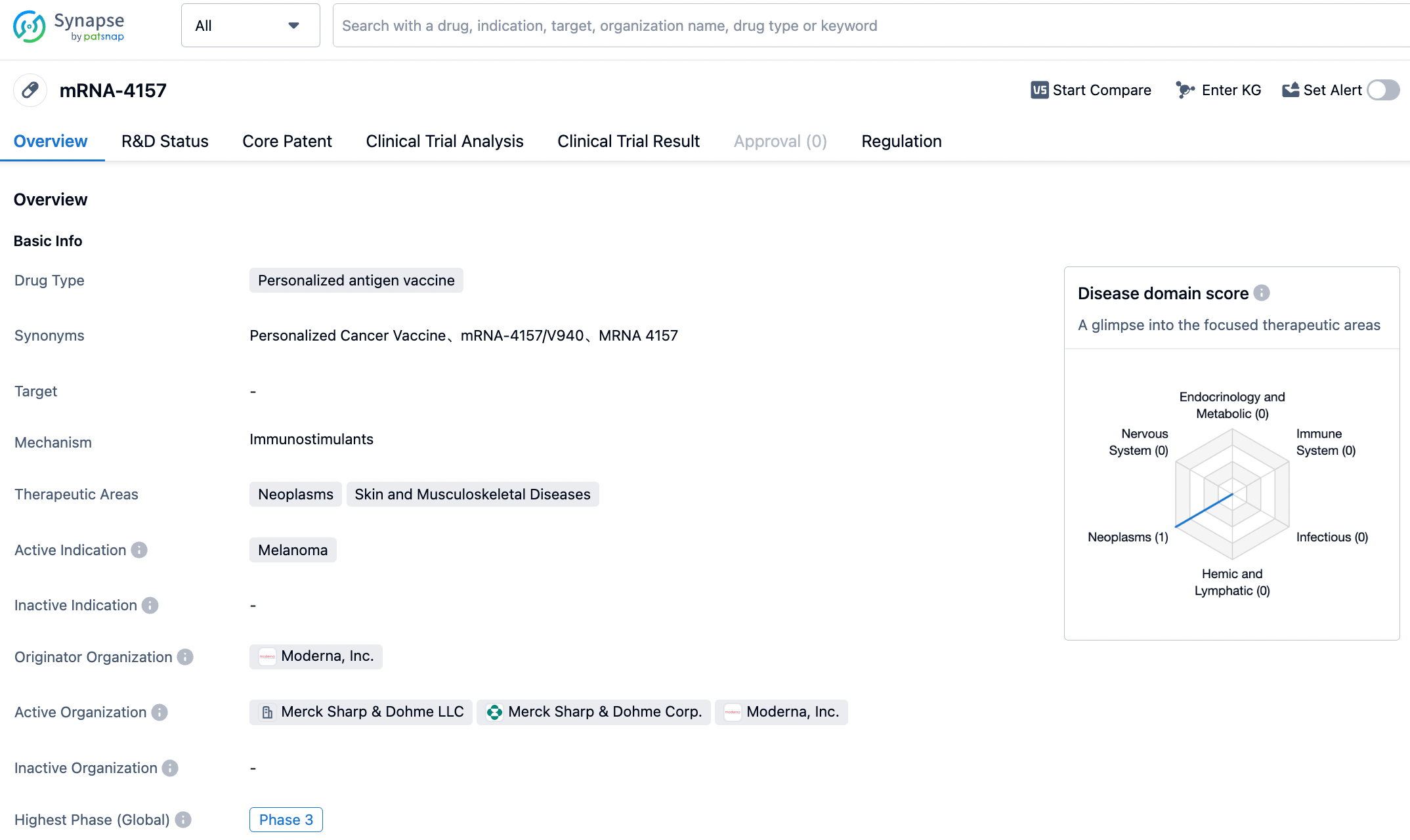
In melanoma patients, compared with Pembrolizumab monotherapy, the vaccine combined with Pembrolizumab treatment reduced the risk of recurrence or death by 44%, reaching the primary endpoint of Relapse-Free Survival (RFS).
These two companies expect to initiate Phase III clinical studies of this vaccine this year and swiftly expand the pipeline to other types of cancers, including non-small cell lung cancer.
As a personalized cancer vaccine, mRNA-4157 targets the patient's specific mutations, encoding up to 34 neoantigens. By activating cancer neoantigen-specific T cells, mRNA-4157 can kill tumor cells. Based on the revealed data, mRNA-4157 seems to have a high chance of winning.
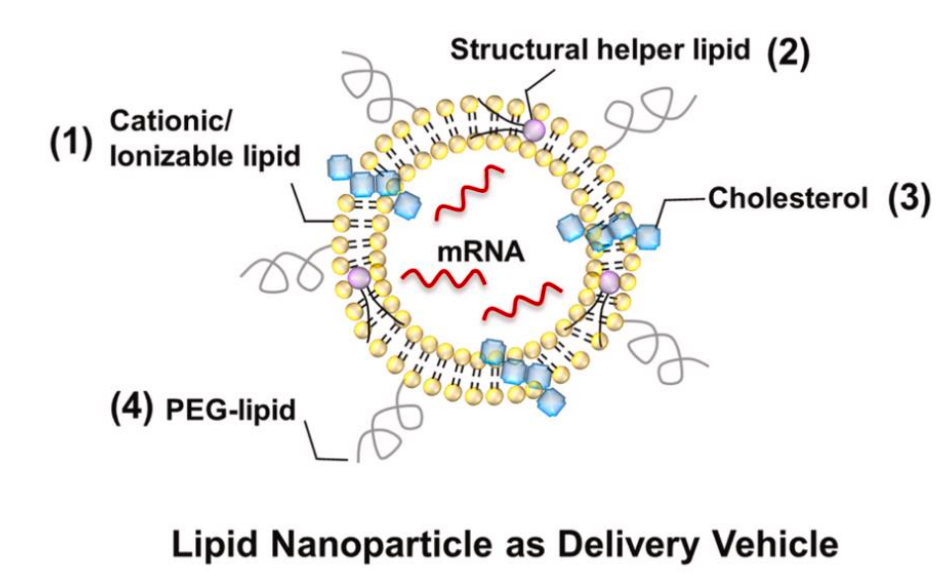
Compared with other types of vaccines, mRNA vaccines can deliver multiple antigens simultaneously, activating both humoral and cell-mediated immune responses. It is widely known that targeting tumor antigens is a prerequisite for a vaccine to work. If the immunogenicity or specificity of the tumor antigens selected by the vaccine is not strong enough, it will be very difficult to succeed.
moderna cancer vaccine pipeline Technology Platform
Moderna possesses a technology platform that spans from research discovery to early development. The research platform, SOFTWAREOF LIFE™, allows scientists to advance ideas to the development stage, providing support services for this process, including equipment capable of rapidly and massively producing mRNA, enabling scientists to conduct a large number of experiments.
The mRNA DesignStudio™ empowers the design of mRNA sequences using algorithms. This platform is capable of reverse simulating mRNA sequences by targeting proteins, and automatically optimizing them through the company's bioinformatics algorithms. Scientists can also manually edit to change mRNA sequences. With the accumulation of a large amount of data, the mRNA Design Studio™ can continuously upgrade and evolve the mRNA design algorithm, providing greater help for subsequent research and development.
The company's early development platform, mRNA EARLY DEVELOPMENT ENGINE™, is capable of providing capabilities such as process development, GLP toxicology research, global regulatory communication, clinical trial preparation and operation. The platform continues to advance candidate drugs to the GLP toxicology and Human Proof of Concept (PoC) stage.
Currently, Moderna has a cGMP clinical center in Norwood, Massachusetts, covering an area of about 200,000 square feet, capable of conducting over 100 batches of cGMP annually, capable of conducting GLP toxicology research as well as Phase I and II clinical trials. At the same time, the company has centralized the necessary elements for production in Norwood, including the production of raw materials and active pharmaceutical ingredients (API), formulation design, filling and other capabilities. This clustered advantage enables R&D work to advance rapidly.
The current prospects for the application of mRNA cancer vaccines have also reached a certain consensus in the industry - for inoperable tumors; for the prevention of recurrence after surgery and chemotherapy. Developers are combining various means to push mRNA cancer vaccines to the forefront of immunotherapy, thereby playing a better role. However, it must be acknowledged that there is still a considerable distance to go before the product is truly launched. We look forward to the early listing of Moderna's mRNA vaccine to benefit tumor patients.