Analysis on the Clinical Research Progress of Akt inhibitors
Akt, also known as protein kinase B, is a crucial enzyme that plays a significant role in various cellular processes within the human body. It is a key mediator of cell survival, growth, and metabolism. Akt is involved in regulating multiple signaling pathways, including those related to cell proliferation, apoptosis, and glucose metabolism. It promotes cell survival by inhibiting apoptosis and stimulating cell growth and proliferation. Akt also regulates glucose metabolism by promoting glucose uptake and utilization. Dysregulation of Akt signaling has been implicated in various diseases, including cancer, diabetes, and neurodegenerative disorders. Understanding the role of Akt is essential for developing targeted therapies in the pharmaceutical industry.
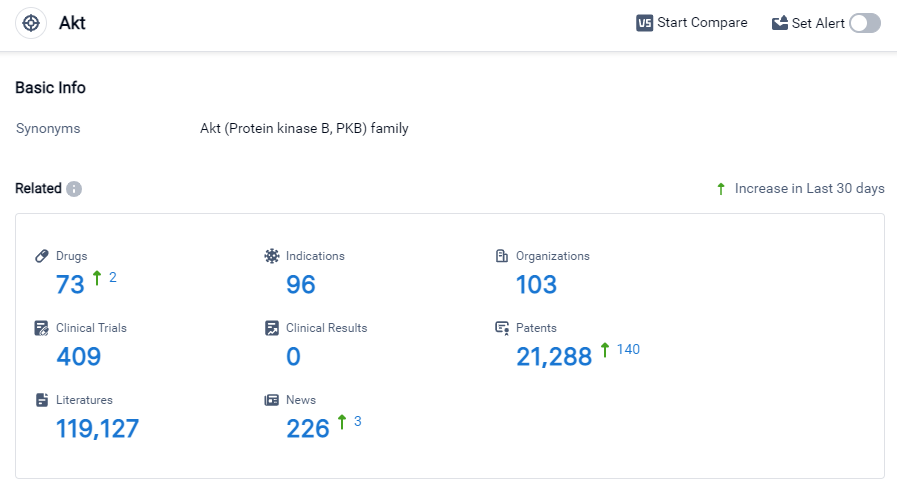
Akt Competitive Landscape
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 73 Akt drugs worldwide, from 103 organizations, covering 96 indications, and conducting 409 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the data, AstraZeneca PLC, Roche Holding AG, and Otsuka Holdings Co., Ltd. are the companies growing fastest under the current target Akt. AstraZeneca PLC has the highest stage of development with drugs in multiple phases. Several drugs under the target Akt have been approved for relevant indications, including breast cancer, prostatic cancer, neoplasms, ovarian cancer, colorectal cancer, and lung cancer. Small molecule drugs and synthetic peptides are progressing rapidly under this target. China is showing progress in the development of drugs under the target Akt, with drugs in phase 3 and preclinical stages. The competitive landscape for target Akt is dynamic, with multiple companies and countries actively involved in research and development. The future development of target Akt is expected to continue with a focus on advancing drugs through clinical trials and expanding indications for approved drugs.
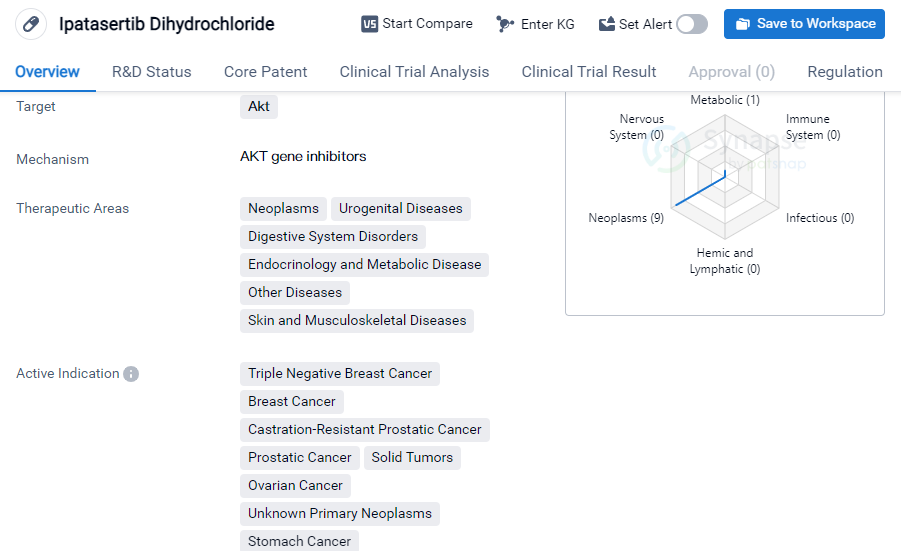
Key drug:Ipatasertib Dihydrochloride
Ipatasertib Dihydrochloride is a small molecule drug that targets Akt, a protein involved in cell survival and growth. It has shown potential therapeutic benefits in various therapeutic areas including neoplasms, urogenital diseases, digestive system disorders, endocrinology and metabolic disease, other diseases, and skin and musculoskeletal diseases.
In terms of active indications, Ipatasertib Dihydrochloride has demonstrated efficacy in treating breast cancer, castration-resistant prostatic cancer, prostatic cancer, solid tumors, ovarian cancer, stomach cancer, gastrointestinal neoplasms, etc. This wide range of indications suggests its potential as a versatile treatment option for different types of cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug was developed by Array BioPharma, Inc., a pharmaceutical company specializing in the discovery, development, and commercialization of targeted small molecule drugs. It has reached phase 3 globally, which is the highest phase of clinical development. Phase 3 trials are the final stage of clinical testing before seeking regulatory approval, indicating that Ipatasertib Dihydrochloride has progressed significantly in its development process.
Furthermore, Ipatasertib Dihydrochloride has been designated as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients. This regulatory status provides certain incentives and benefits to the drug developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
In summary, Ipatasertib Dihydrochloride is a small molecule drug developed by Array BioPharma, Inc. that targets Akt. It has shown promise in treating various types of cancers and has reached the advanced stage of phase 3 clinical trials globally. Its designation as an orphan drug highlights its potential to address unmet medical needs in rare diseases.
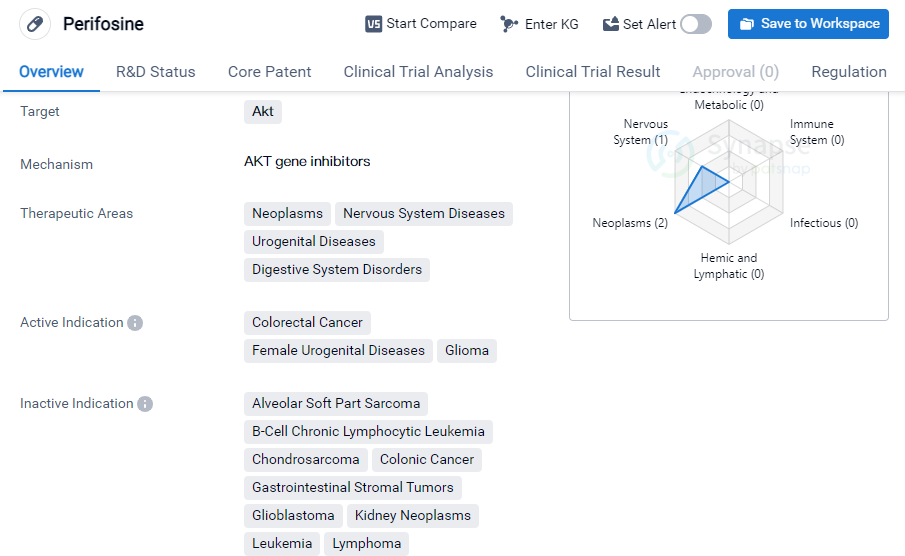
Perifosine
Perifosine is a small molecule drug that is being developed by AEterna Zentaris GmbH. It is designed to target Akt, a protein that plays a role in cell survival and proliferation. The drug is currently in phase 3 of clinical trials, which is the final stage before seeking regulatory approval.
Perifosine has shown potential in treating a range of therapeutic areas, including neoplasms (abnormal growth of cells), nervous system diseases, urogenital diseases, and digestive system disorders. Specifically, it has demonstrated efficacy in treating colorectal cancer, female urogenital diseases, and glioma, a type of brain tumor.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
One notable aspect of Perifosine is its designation as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the drug developer, such as extended market exclusivity and financial support for clinical trials.
The fact that Perifosine has reached phase 3 of clinical trials indicates that it has shown promising results in earlier stages of testing. Phase 3 trials involve a larger number of participants and aim to further evaluate the drug's safety and effectiveness. If the results continue to be positive, AEterna Zentaris GmbH may seek regulatory approval to bring Perifosine to market.
In summary, Perifosine is a small molecule drug developed by AEterna Zentaris GmbH that targets Akt. It has shown potential in treating neoplasms, nervous system diseases, urogenital diseases, and digestive system disorders. The drug is currently in phase 3 of clinical trials and has demonstrated efficacy in treating colorectal cancer, female urogenital diseases, and glioma. Its designation as an orphan drug provides certain benefits and incentives to the developer.