Analysis on the Research Progress of Oncolytic virus
Oncolytic virus (OVs) refers to a generic name for viruses that naturally exist or are genetically edited to selectively kill tumor cells. In principle, the physiological metabolic environment inside tumor cells is different from that of normal cells. Oncolytic viruses, due to their characteristics, can infect tumor cells and replicate selectively within tumor cells, leading to the dissolution and death of tumor cells. At the same time, they cannot or can only replicate in small amounts within normal cells, avoiding the impact on normal cells.
Oncolytic virotherapy is a method that uses the characteristics of oncolytic viruses as an anticancer therapy. Oncolytic virotherapy has high tumor specificity, good effects, minor side effects, and broad prospects for development. Moreover, there are precedents approved by regulatory authorities.
Advantages of Oncolytic Virotherapy
1. Oncolytic viruses have natural synergies with various anti-cancer drugs and therapies (including chemotherapy drugs, targeted therapies, cell therapies). They can turn "cold tumors", which are unresponsive to other immune therapies, into "hot tumors" that can respond to drugs. The combined use of both does not have obvious cumulative toxicity, thus creating a more advanced cancer treatment method. Main indications are melanoma, glioma, breast cancer, non-small cell lung cancer, colon cancer, bladder cancer, head and neck cancer, and prostate cancer.
2. The oncolytic virus can be modulated and controlled to limit its impact on normal cells, hence this therapy is expected to have minor side effects. Existing clinical data indicates that oncolytic viruses overall demonstrate better safety compared to other therapies.
3. The modification of oncolytic viruses has multiple possibilities, which can further enhance their killing power against cancer cells. For example, the virus can be modified to deliver tumor suppressor genes, or add immune regulation functions.
4. Oncolytic virotherapy is applicable to the majority of solid tumors.
Due to its uniqueness, oncolytic virotherapy has drawn wide attention. However, it is still in its early stages of development. With both opportunities and challenges, it is expected that this therapy will play an important role in the treatment of solid tumors.
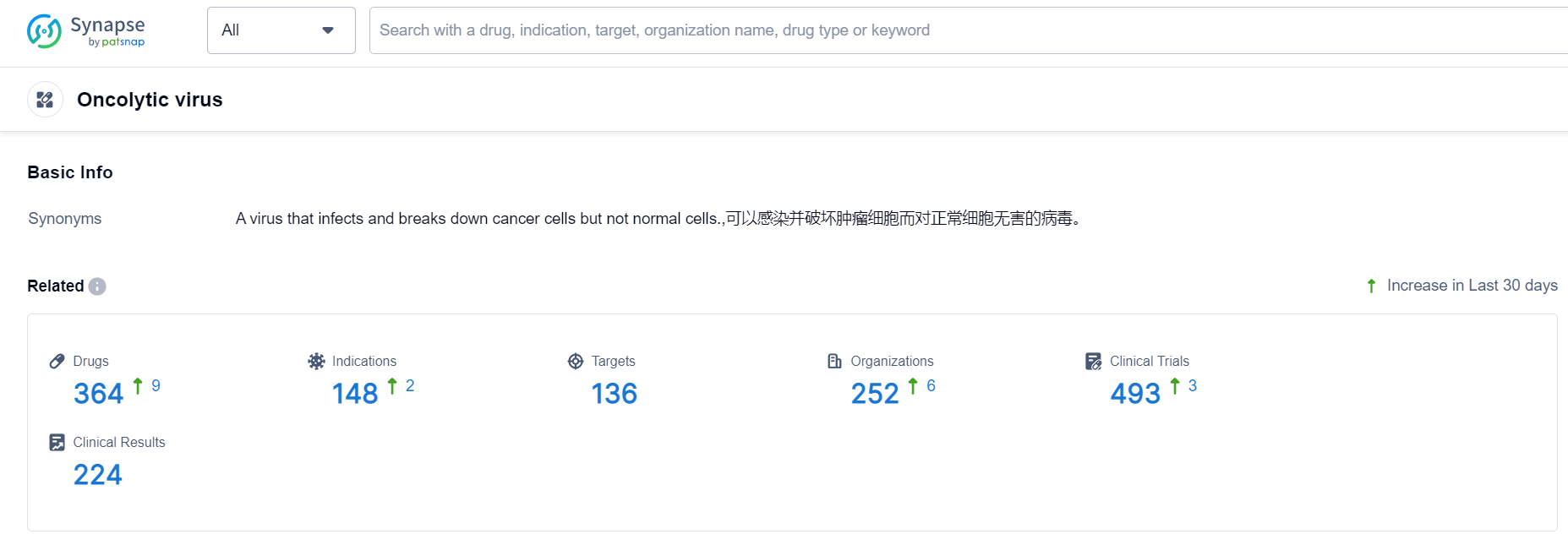
Oncolytic virus Competitive Landscape
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 364 Oncolytic virus drugs worldwide, from 252 organizations, covering 136 targets, 148 indications, and conducting 493 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
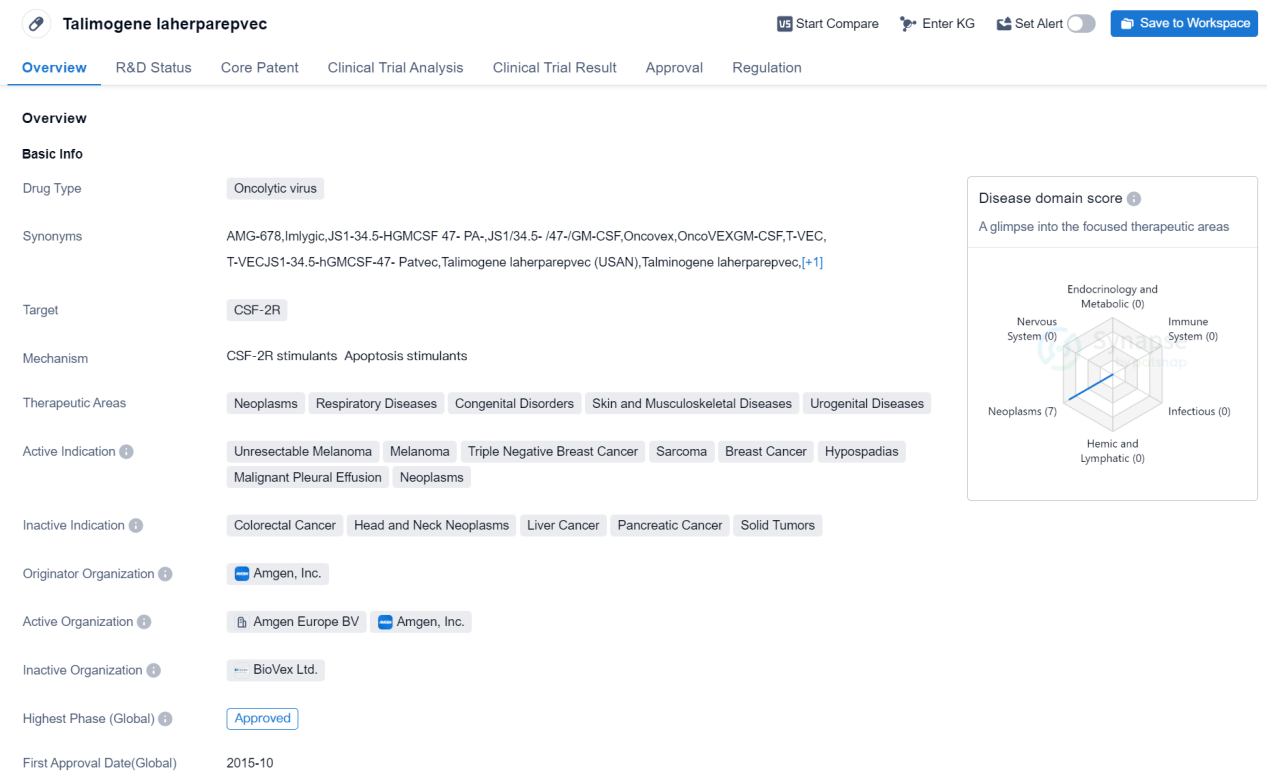
Approved Oncolytic virus Medicinal: Talimogene laherparepvec
Talimogene laherparepvec is an oncolytic virus drug that targets CSF-2R. It has been approved for use in the treatment of various therapeutic areas including neoplasms, respiratory diseases, congenital disorders, skin and musculoskeletal diseases, and urogenital diseases. The active indications for this drug include unresectable melanoma, melanoma, triple negative breast cancer, sarcoma, breast cancer, hypospadias, malignant pleural effusion, and neoplasms.
The drug was developed by Amgen, Inc., a renowned pharmaceutical company in the United States. It received its first approval in October 2015 in the United States. Talimogene laherparepvec is regulated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
As an oncolytic virus, Talimogene laherparepvec works by infecting and destroying cancer cells while leaving healthy cells unharmed. It is administered through injection directly into the tumor, allowing the virus to replicate and spread within the tumor, leading to its destruction. This targeted approach offers a promising treatment option for patients with unresectable melanoma, melanoma, triple negative breast cancer, sarcoma, and breast cancer.
The approval of Talimogene laherparepvec marks a significant milestone in the field of biomedicine. It provides healthcare professionals with a new tool to combat various types of cancer, particularly those that have been difficult to treat using conventional therapies. The drug's orphan drug status highlights its potential to address unmet medical needs and improve outcomes for patients with rare diseases.
Since its approval, Talimogene laherparepvec has been used in clinical practice, demonstrating its safety and efficacy. Ongoing research and clinical trials are further exploring its potential applications in other therapeutic areas and indications.
In conclusion, Talimogene laherparepvec is an approved oncolytic virus drug developed by Amgen, Inc. It targets CSF-2R and has shown effectiveness in treating various types of cancer, including unresectable melanoma, melanoma, triple negative breast cancer, sarcoma, and breast cancer. Its orphan drug status reflects its potential to address unmet medical needs. The drug's approval in 2015 marked a significant advancement in the field of biomedicine, offering a targeted and promising treatment option for patients with challenging-to-treat cancers. Ongoing research continues to explore its potential applications in other therapeutic areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
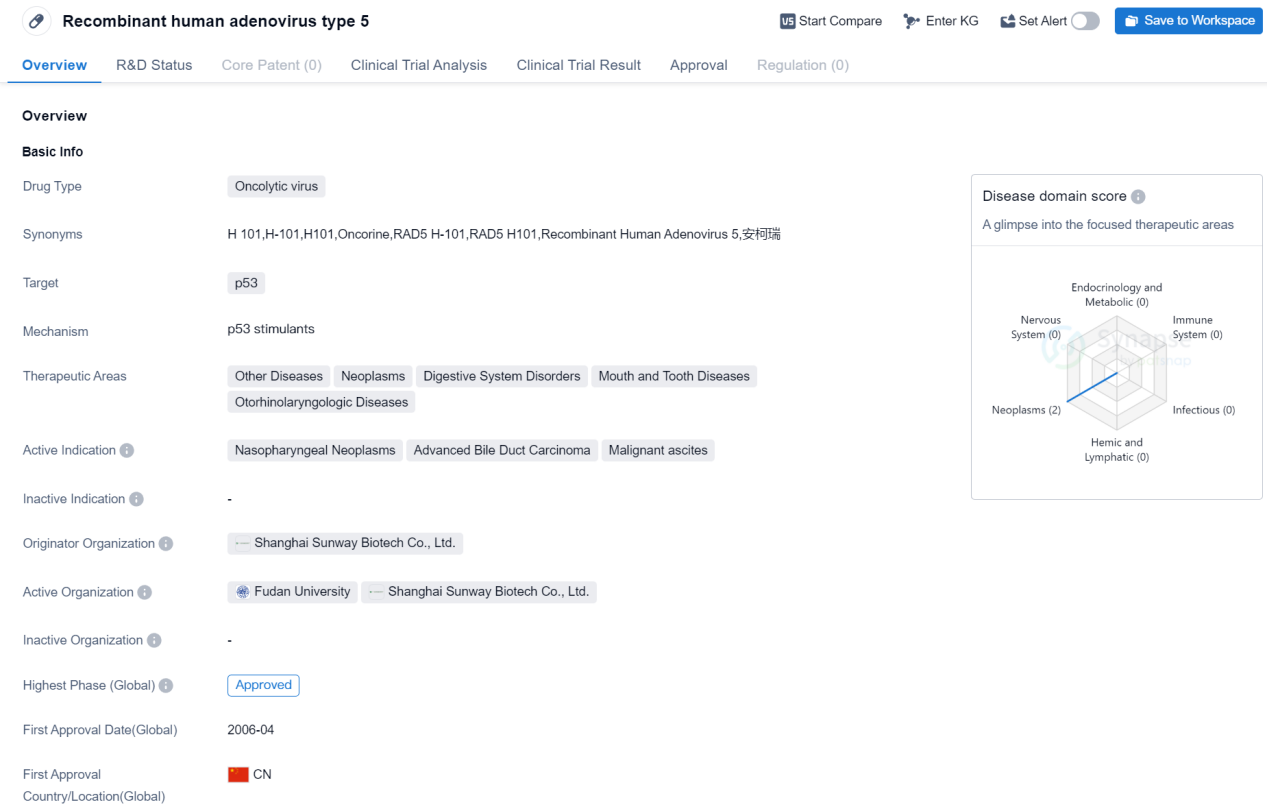
Recombinant human adenovirus type 5
The drug Recombinant human adenovirus type 5 is classified as an oncolytic virus, meaning it is designed to specifically target and destroy cancer cells. The drug's primary target is the p53 protein, which is involved in regulating cell growth and preventing tumor formation. By targeting p53, this drug aims to inhibit the growth and spread of cancer cells.
Recombinant human adenovirus type 5 is indicated for the treatment of various diseases, including neoplasms (tumors), digestive system disorders, mouth and tooth diseases, and otorhinolaryngologic diseases. Specifically, it has shown efficacy in treating nasopharyngeal neoplasms, advanced bile duct carcinoma, and malignant ascites.
The drug was developed by Shanghai Sunway Biotech Co., Ltd., an originator organization based in China. It has received approval for use globally. The first approval for this drug was granted in China in April 2006.
As an oncolytic virus, Recombinant human adenovirus type 5 has shown promise in the field of biomedicine. By specifically targeting p53 and focusing on various therapeutic areas, it has the potential to provide effective treatment options for patients with different types of cancers and related diseases. The drug's approval in China indicates its safety and efficacy profile has been evaluated and deemed acceptable for use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Recombinant human adenovirus type 5 represents a significant advancement in the pharmaceutical industry's efforts to combat cancer and related diseases. Its ability to selectively target cancer cells and its approval in multiple therapeutic areas make it a valuable addition to the treatment options available to patients. Continued research and development in this field may further enhance the drug's effectiveness and expand its applications in the future.