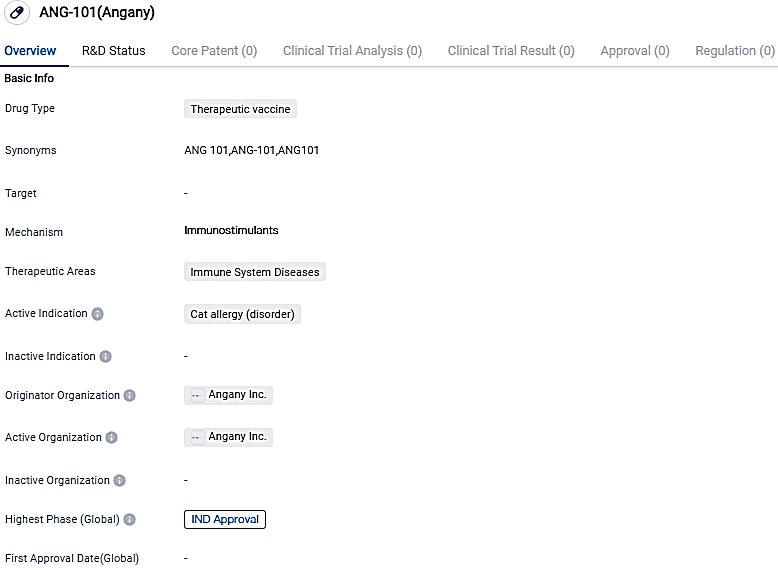
Angany reveals initial human clinical trial for ANG-101, a potential vaccine for feline allergies
Angany Inc.. has revealed that it has obtained approval from MHRA to carry out the initial clinical trial of its vaccine candidate ANG-101 aimed at combating allergies in humans to cats. This clinical investigation is a first-of-its-kind, open label and singular location examination of the vaccine's safety, allergenicity, and immunogenic potential in patients who are allergic to cat dander. The study will be conducted under the supervision of Professor Stephen Durham and Dr Guy Scadding, both are top clinical allergy experts from Imperial College London.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
At present, there is no permanent solution for cat allergy, which is a ubiquitous and perpetual health issue. Drugs bought over the counter and with prescriptions can only bring temporary relief to the patients, but they do not hinder the further development of the allergy.
The sole procedure that can modify the disease is desensitization which involves allergen immunotherapy. However, this procedure requires long-term application of cat allergens and is frequently bound with a low success rate and the jeopardy of allergic reactions.
Angany’s ANG-101 serves as a revolutionary solution to cat allergies by altering its progression. It is a therapeutic vaccine that is an offshoot of its unique eBioparticle-Potentiated Immunotherapy™ technology.
The dynamic component for immunotherapy in ANG-101 is an enveloped bioparticle that is distinctive in its dimensions, being 140 nm, and its form, similar to a virus, having its surface coated with thousands of the basic cat allergen replicas.
"We are exceedingly glad to have our innovative and forthcoming strategy for cat allergy immunotherapy be tested on patients. This is the first phase in a detailed clinical development scheme," says the company.
The trial is named “HOPE” as this crucial phase in the vaccine development program of Angany gives a new prospect for the countless individuals suffering from allergies globally. ANG-101 is the pioneer in a group of vaccines being developed to address all major allergies in human beings and their pets.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
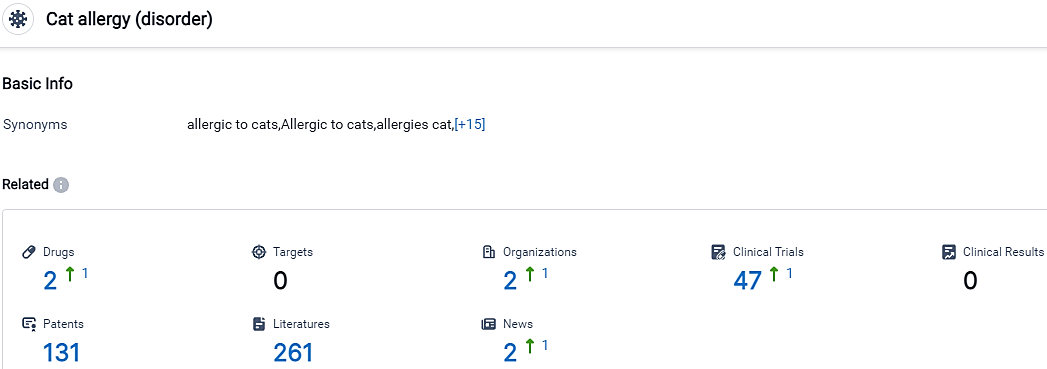
According to the data provided by the Synapse Database, As of October 17, 2023, there are 2 investigational drugs for the Cat allergy, including 2 R&D institutions involved, with related clinical trials reaching 47,and as many as 131 patents.
Cat allergies manifest as various symptoms such as skin diseases and respiratory diseases, and there are different anti allergic products available on the market based on these symptoms. Although there are more and more pet allergy products, the mainstream products are still therapeutic drugs, such as anti-inflammatory drugs, antibiotics, etc. Although the cat allergy vaccine market has not yet formed a large-scale market, it is gradually developing with the increasing emphasis on pet health among people. However, there is currently a lack of specialized vaccines for cat allergies in the market, and the majority of the market still focuses on vaccines to prevent major pet diseases.