Advances in Clinical Research on BRAF Inhibitor
BRAF, a protein kinase, plays a crucial role in the human body by regulating cell growth and division. It is a key component of the MAPK signaling pathway, which controls various cellular processes such as proliferation, differentiation, and survival. Mutations in the BRAF gene can lead to abnormal activation of the pathway, resulting in uncontrolled cell growth and the development of certain cancers, particularly melanoma. Targeting BRAF mutations with specific inhibitors has revolutionized the treatment of BRAF-mutant cancers, providing patients with more effective and personalized therapeutic options. Understanding the role of BRAF has significantly advanced our knowledge of cancer biology and paved the way for innovative targeted therapies.
BRAF Competitive Landscape
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 51 BRAF drugs worldwide, from 88 organizations, covering 79 indications, and conducting 1761 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the current competitive landscape of target BRAF reveals that Bayer AG is the leading company in terms of R&D progress, with drugs in multiple phases of development. Several companies, including Roche Holding AG, Pfizer Inc., Novartis AG, and Pierre Fabre Participations SAS, have also made significant progress in their R&D efforts. Drugs under the target BRAF have been approved for various indications, including Melanoma, Colorectal Cancer, Hepatocellular Carcinoma, and Non-Small Cell Lung Cancer. Small molecule drugs, PROTACs, and biosimilars like Monoclonal antibodies and Antibody drug conjugates (ADCs) are progressing rapidly. The United States, European Union, Japan, and China are the countries/locations with the highest development activity. China has shown notable progress in the development of drugs under the target BRAF. Overall, the target BRAF presents a competitive landscape with multiple companies and drug types vying for success in various indications and countries/locations.
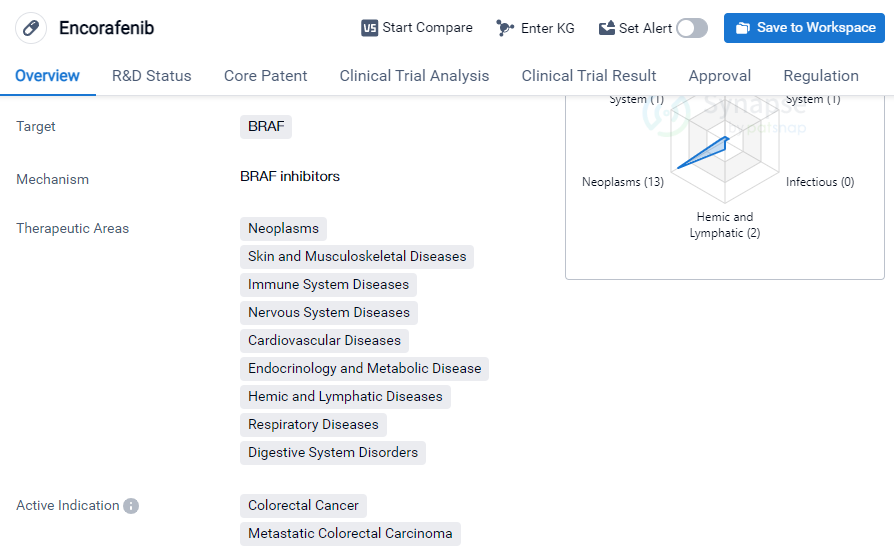
Key drug: Encorafenib
Encorafenib is a small molecule drug that primarily targets the BRAF protein. It has been developed by Array BioPharma, Inc. and has received approval for use in various therapeutic areas. These areas include neoplasms, skin and musculoskeletal diseases, immune system diseases, nervous system diseases, cardiovascular diseases, etc.
The drug has shown efficacy in treating several types of cancer, including colorectal cancer, metastatic colorectal carcinoma, metastatic melanoma, melanoma, BRAF V600E mutant non-small cell lung cancer, etc.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Encorafenib has achieved the highest phase of development, which is approved globally. In China, it is currently in phase 3 of development. The drug received its first approval in the United States in June 2018. It is worth noting that Encorafenib is regulated as an orphan drug, indicating its potential to treat rare diseases or conditions.
In summary, Encorafenib is a small molecule drug developed by Array BioPharma, Inc. It targets the BRAF protein and has been approved for use in various therapeutic areas, including multiple types of cancer. It has achieved the highest phase of development globally and is currently in phase 3 in China. The drug received its first approval in the United States in 2018 and is regulated as an orphan drug.
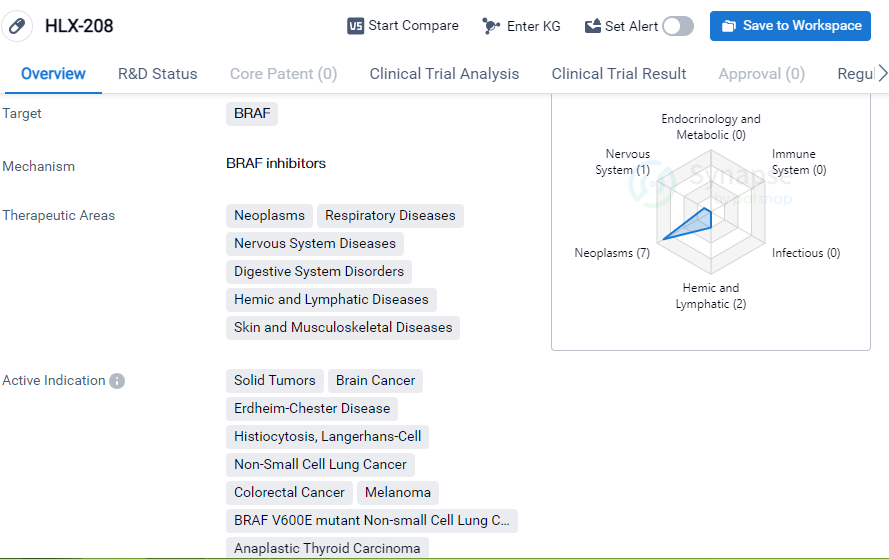
HLX-208
HLX-208 is a small molecule drug that targets BRAF, a protein involved in cell signaling pathways. It is being developed by Suzhou NeuPharman Co. Ltd., a pharmaceutical company specializing in biomedicine. The drug is currently in phase 3 of clinical trials globally, indicating that it has progressed through earlier stages of development and is now being tested for its safety and efficacy in a larger population.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
HLX-208 has shown potential in treating a wide range of therapeutic areas, including neoplasms (abnormal growth of cells), respiratory diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, and skin and musculoskeletal diseases. This suggests that the drug may have a broad spectrum of activity and could be beneficial in various medical conditions.
The active indications for HLX-208 include solid tumors, brain cancer, Langerhans-cell histiocytosis, non-small cell lung cancer, colorectal cancer, melanoma, BRAF V600E mutant non-small cell lung cancer, and anaplastic thyroid carcinoma. These indications cover a range of cancer types, indicating that HLX-208 may have potential as an anti-cancer therapy.
One notable aspect of HLX-208 is its regulatory status as a breakthrough therapy. Breakthrough therapy designation is granted by regulatory authorities, such as the U.S. Food and Drug Administration (FDA), to expedite the development and review of drugs that show substantial improvement over existing treatments for serious or life-threatening conditions. This designation highlights the potential of HLX-208 to address unmet medical needs and suggests that it may have significant clinical benefits.
In summary, HLX-208 has shown potential in treating various therapeutic areas, particularly in the field of oncology, with active indications including solid tumors, brain cancer, and non-small cell lung cancer. Its breakthrough therapy designation further emphasizes its potential as an innovative and promising treatment option.