Annexon Announces Phase 3 Success of ANX005 for Guillain-Barré Syndrome
Annexon, Inc., a biopharmaceutical company dedicated to developing an advanced platform of innovative therapies aimed at treating severe classical complement-mediated neuroinflammatory conditions affecting the body, brain, and eyes, has reported favorable top-line results from its pivotal Phase 3 randomized placebo-controlled trial involving patients with Guillain-Barré syndrome. The Phase 3 trial successfully met its primary endpoint, with a highly statistically significant 2.4-fold improvement on the GBS-disability scale at week 8 for patients receiving ANX005 at a dose of 30 mg/kg.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Guillain-Barré Syndrome (GBS) is an acute, rapidly progressing neurological disorder with a limited therapeutic window, leading to the hospitalization of over 22,000 individuals annually in the United States and Europe. The substantial and enduring disease burden of GBS on patients, caregivers, healthcare facilities, and insurers results in a multi-billion-dollar annual economic impact on the U.S. healthcare system. Presently, the U.S. Food and Drug Administration (FDA) has not approved any treatments for GBS.
Douglas Love, president and CEO of Annexon, stated, "In our Phase 3 trial, early administration of ANX005 provided swift neuroprotection, halting disease progression and aiding in quicker recovery for GBS patients. These findings bolster Annexon’s core hypothesis that inhibiting C1q is a potent mechanism to arrest neuroinflammation and highlight the potential of ANX005 and our classical complement platform in treating GBS and numerous other diseases affecting the body, brain, and eye."
The randomized, placebo-controlled Phase 3 trial included 241 participants from Bangladesh and the Philippines, assessing two doses of ANX005, 30 mg/kg and 75 mg/kg. Both doses achieved rapid and complete suppression of complement activity but differed in the duration of C1q inhibition—the 30 mg/kg dose lasted one week, while the 75 mg/kg dose lasted two to three weeks.
ANX005 75 mg/kg surpassed the placebo on multiple measures, although it did not attain statistical significance on the primary endpoint of GBS-DS at week eight. The two doses were appraised based on insights from an earlier Phase 1b proof-of-concept study, which showed efficacy in combined analyses of both short and long durations of ANX005 C1q inhibition.
Given that classical complement causes tissue damage in the early stages of disease but aids in nerve repair after acute injury, the promising Phase 3 results with the 30 mg/kg dose, leading to one week of C1q inhibition, seemed to establish the optimal treatment timeframe.
The clinical safety and tolerability assessments of ANX005 at both doses in the Phase 3 study indicated a generally well-tolerated profile, with no new safety signals. Most adverse events were mild to moderate, classified as Grade 1 or 2. The most common treatment-related adverse events were mild rash due to infusion reactions. No autoimmune-related adverse events or drug-related deaths or severe infections were noted.
ANX005 has received Fast Track and Orphan Drug Designations from the FDA. Additionally, it has been granted Orphan Drug Designation by the European Medicines Agency based on meta-analyses of previous studies with ANX005 and IVIg, which demonstrated significant early muscle strength improvements with ANX005, translating into notable health gains, including reduced need for mechanical ventilation.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
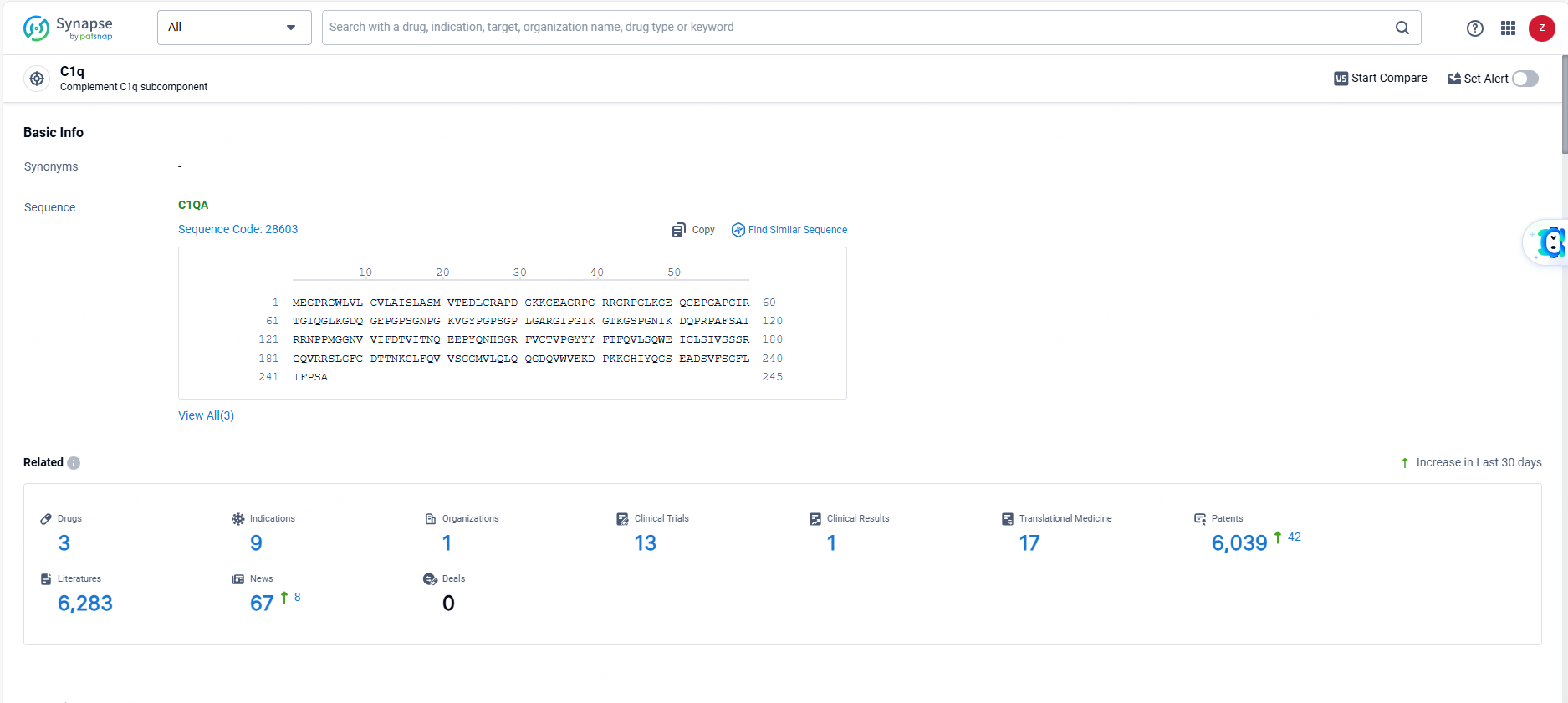
According to the data provided by the Synapse Database, As of June 13, 2024, there are 3 investigational drugs for the C1q targets, including 9 indications, 1 R&D institutions involved, with related clinical trials reaching 13, and as many as 6039 patents.
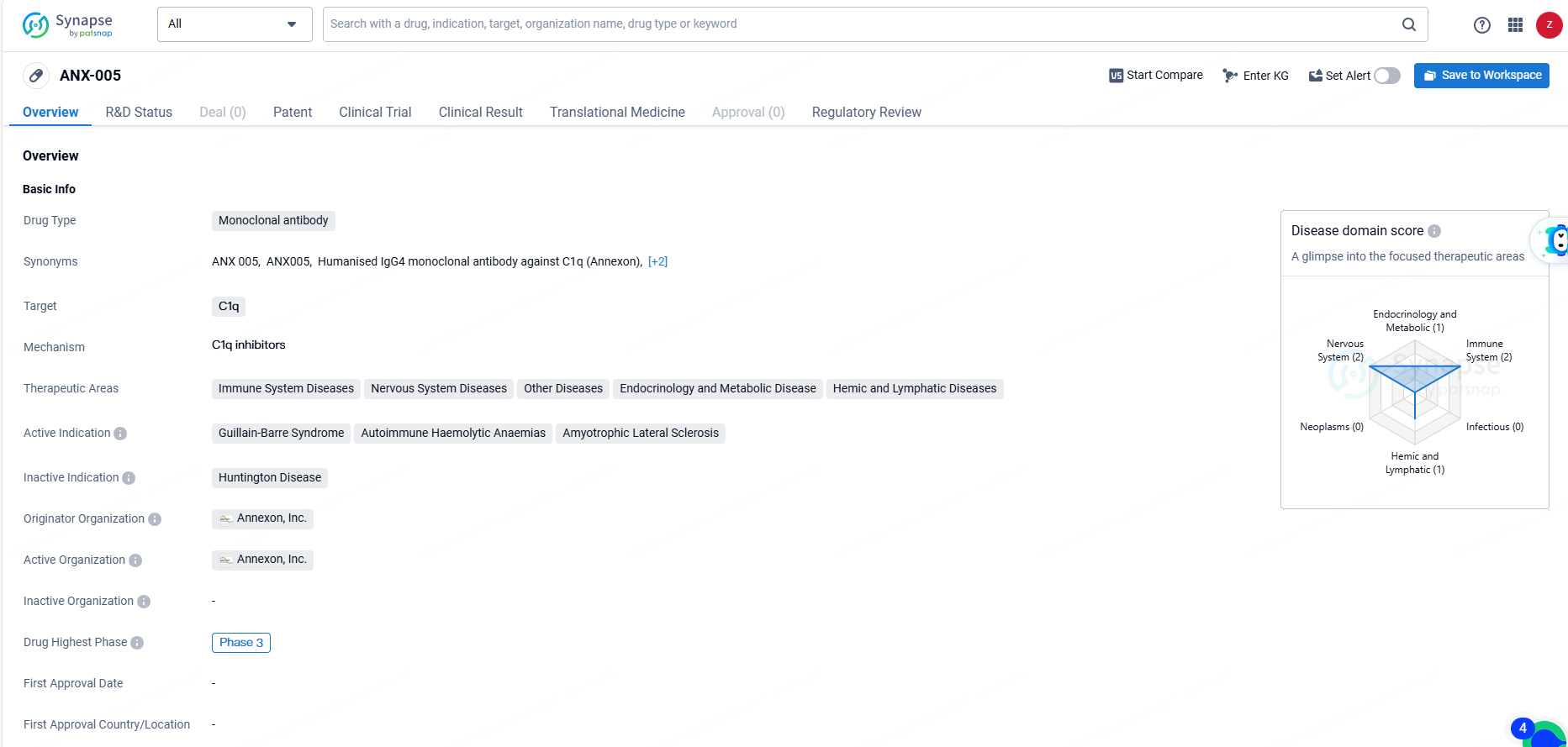
ANX-005 is a monoclonal antibody drug developed by Annexon, Inc. with a focus on targeting C1q for the treatment of various therapeutic areas and active indications. The drug has reached Phase 3 of development and has been granted Fast Track and Orphan Drug designations, highlighting its potential to address unmet medical needs for rare diseases.