Arcturus Therapeutics gets FDA approval for ARCT-032, an inhaled mRNA treatment for cystic fibrosis
Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT), an international messenger RNA medicine enterprise aimed at creating vaccines for infectious diseases and tackling unmet needs in liver and respiratory rare conditions, announced that the U.S. Food and Drug Administration (FDA) has given a “Study May Proceed” notification for the Company’s Investigational New Drug (IND) application, ARCT-032, for the treatment of cystic fibrosis (CF). This FDA approval of the ARCT-032 IND application allows the Company to commence a Phase 2 multiple ascending dose study to assess the safety, tolerability, and efficacy of ARCT-032 in individuals with cystic fibrosis.
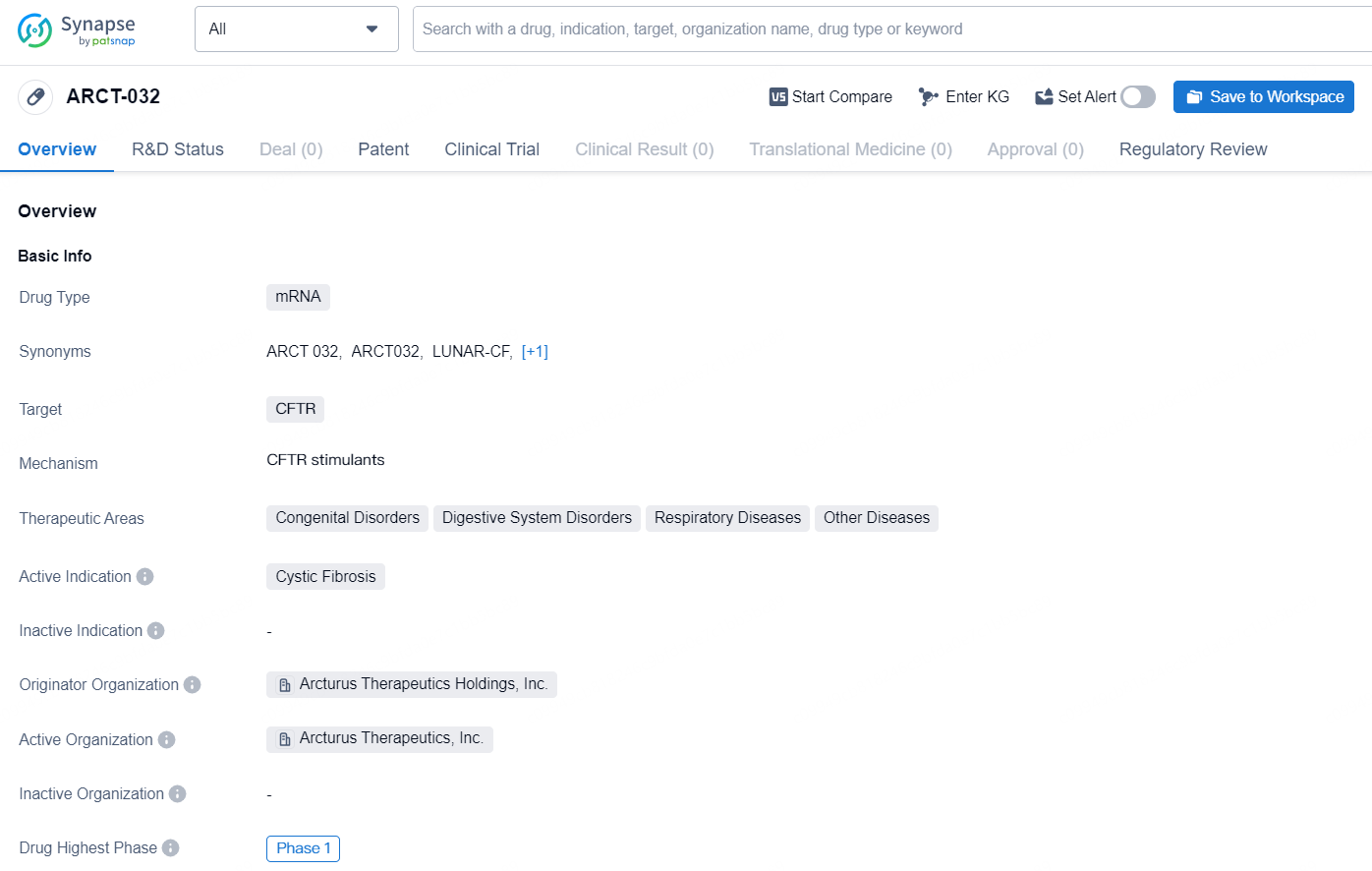
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Juergen Froehlich, Chief Medical Officer of Arcturus Therapeutics, stated, "The Phase 2 Study May Proceed notification enables us to explore ARCT-032 as a possible treatment for cystic fibrosis (CF) patients, additionally providing a chance to further substantiate our LUNAR® technology for mRNA delivery via inhalation. The study will assess the safety and efficacy of ARCT-032 given over several weeks at various dose levels in CF patients who either do not qualify for or gain no benefit from CFTR modulator therapy."
Cystic fibrosis, a globally prevalent and life-limiting disease, arises from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, resulting in decreased or absent CFTR protein and/or function in the respiratory tract. This deficiency affects chloride transport, essential for maintaining airway surface homeostasis, leading to thicker mucus that is harder to clear. Consequently, this causes airway blockages and predisposes patients to infection, inflammation, respiratory failure, and other severe complications. Standard treatment for many CF patients involves CFTR modulators, however, around 40,000 individuals in the U.S. and over 105,000 globally live with CF. About 15% of these patients do not respond to CFTR modulators due to either non-functional or missing CFTR proteins or drug intolerance.
ARCT-032, an inhaled investigational mRNA therapeutic, aims to produce functional CFTR in the lungs of CF patients. It has received Orphan Medicinal Product Designation from the European Medicines Agency (EMA) and Orphan Drug Designation alongside Rare Pediatric Disease Designation from the U.S. Food and Drug Administration (FDA) for treating cystic fibrosis. Utilizing Arcturus’ LUNAR® lipid-mediated aerosolized platform, ARCT-032 delivers CFTR mRNA directly to the lungs. Lung disease is the principal cause of morbidity and mortality in CF patients. Functional CFTR mRNA expression in the lungs of CF patients could potentially restore CFTR activity and alleviate the progressive lung disease. The ARCT-032 program is backed by preclinical data from studies in rodents, ferrets, and primates, demonstrating the restoration of CFTR expression and functionality in human bronchial epithelial cells.
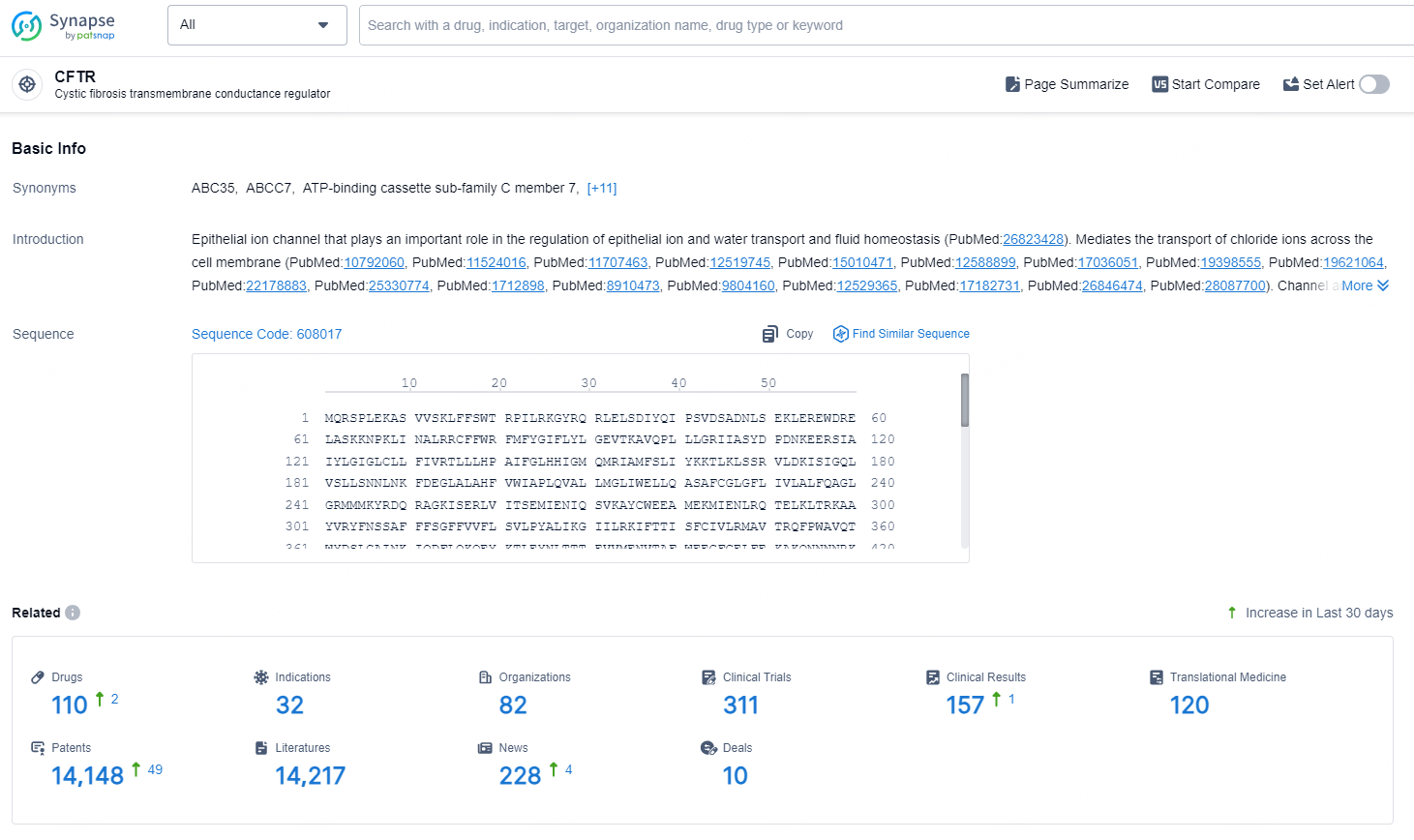
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 4, 2024, there are 110 investigational drugs for the CFTR, including 32 targets, 82 R&D institutions involved, with related clinical trials reaching 311, and as many as 14148 patents.
According to the data provided by the Synapse Database, As of September 4, 2024, there are 151 investigational drugs for the CFTR targets, including 125 indications, 170 R&D institutions involved, with related clinical trials reaching 709, and as many as 10186 patents.