IgGenix Shares Findings on IGNX001 for Peanut Allergy at 2024 ASCIA Meeting
IgGenix, Inc., a company focused on discovering and developing preclinical antibodies with a novel strategy to combat IgE-mediated conditions, revealed promising data about its primary candidate, IGNX001. This candidate is an IgG4 monoclonal antibody therapy designed to treat peanut allergy. The findings were shared at the 2024 Annual Conference of the Australasian Society of Clinical Immunology and Allergy (ASCIA) held in Adelaide, Australia, from September 3-6, 2024.
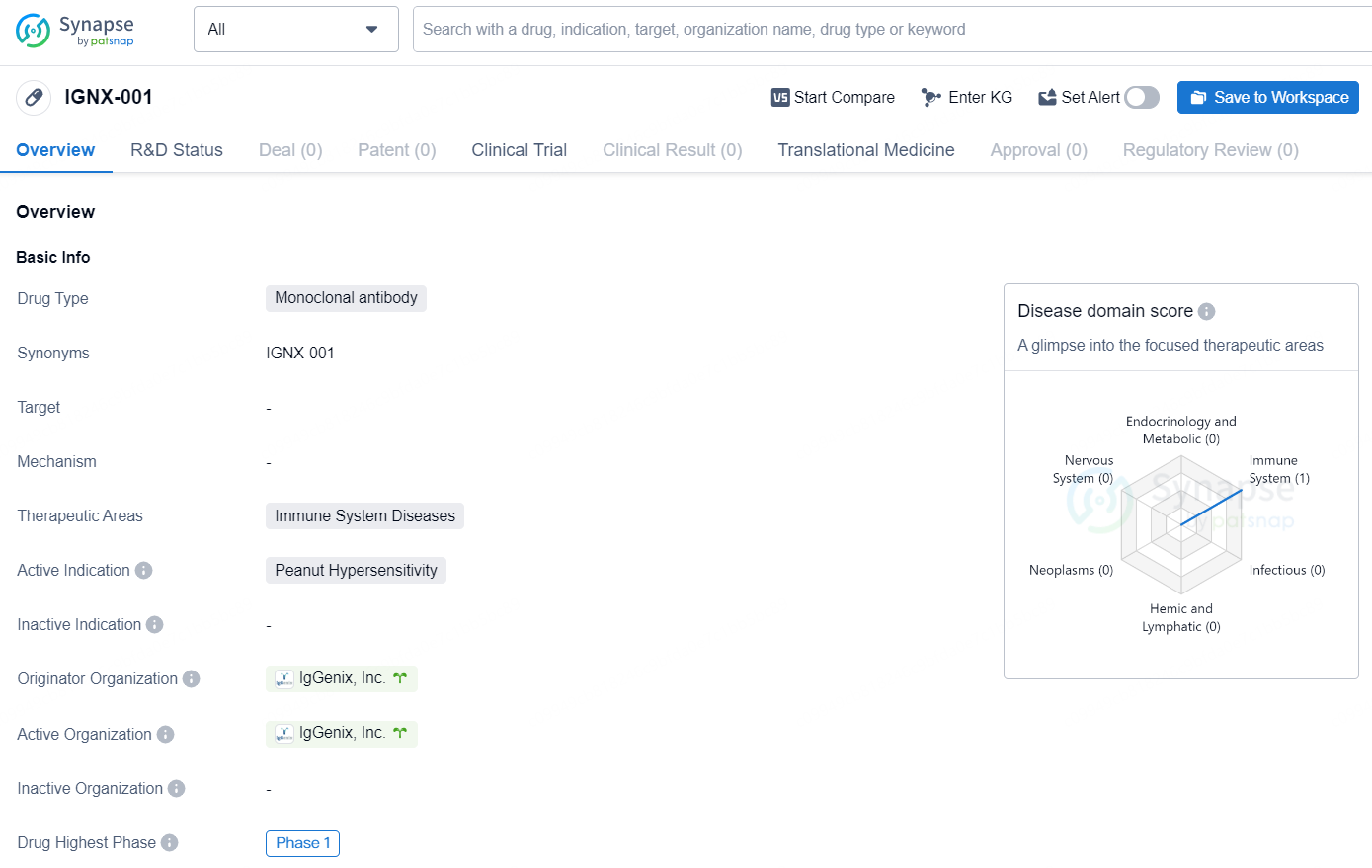
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Michael O’Sullivan, MBBS, FRACP, FRCPA, the principal investigator, shared insights on IgGenix’s Phase 1 human clinical trial for peanut allergies, titled “ACCELERATE Peanut.” This ongoing study is currently in the screening phase and aims to assess the safety and tolerability of IGNX001, a therapeutic candidate designed to provide swift protection for those with peanut allergies.
Derek Croote, PhD, chief technology officer and co-founder of IgGenix, presented preclinical data demonstrating IGNX001’s strong efficacy in preventing peanut-induced mast cell activation and anaphylaxis in a mouse model of peanut allergy. These comprehensive preclinical results were recently published in The Journal of Allergy and Clinical Immunology.
“IGNX001 represents a significant advancement in peanut allergy treatment,” remarked Michael O’Sullivan, MBBS, FRACP, FRCPA, a Consultant Clinical Immunologist at Fiona Stanley Hospital and Perth Children’s Hospital, as well as a Clinical Senior Lecturer at the University of Western Australia. “Leveraging advanced research to convert harmful IgE antibodies into IgG4 blocking antibodies that can bind to allergens without triggering reactions, this innovative approach offers a potentially quicker and more effective alternative to existing therapies. It could revolutionize peanut allergy management by reducing the risk of severe reactions and enhancing the quality of life for millions globally.”
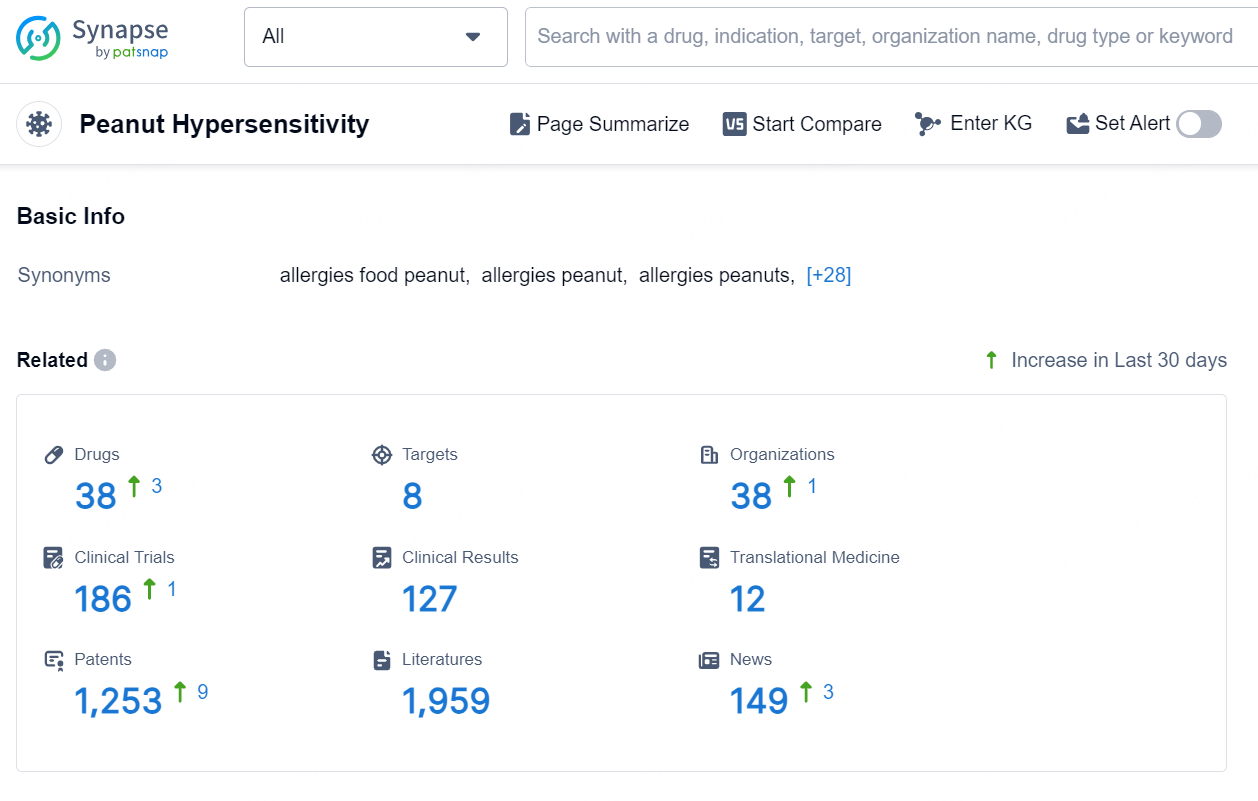
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 4, 2024, there are 38 investigational drugs for the Peanut Hypersensitivity, including 8 targets, 38 R&D institutions involved, with related clinical trials reaching 186, and as many as 1253 patents.
IGNX-001 is a monoclonal antibody drug developed by IgGenix, Inc. The drug is designed to target immune system diseases, with a specific focus on peanut hypersensitivity. As of the latest information available, IGNX-001 has progressed to the highest phase of clinical development, which is Phase 1. Monoclonal antibodies are a type of biological therapy that can target specific proteins on cells. In the case of IGNX-001, the drug is intended to work by modulating the immune system's response to peanuts, potentially offering a new treatment option for individuals with peanut hypersensitivity.