Argenx announces EU approval for subcutaneous VYVGART® to treat Generalized Myasthenia Gravis
argenx SE, an international immunology firm dedicated to enhancing the life quality of those dealing with profound autoimmune disorders, declared that VYVGART (efgartigimod alfa), a subcutaneous injection, has been green-lighted by EC to pair with standard therapy. This is for addressing generalized myasthenia gravis among adult patients exhibiting positive anti-acetylcholine receptor antibody.
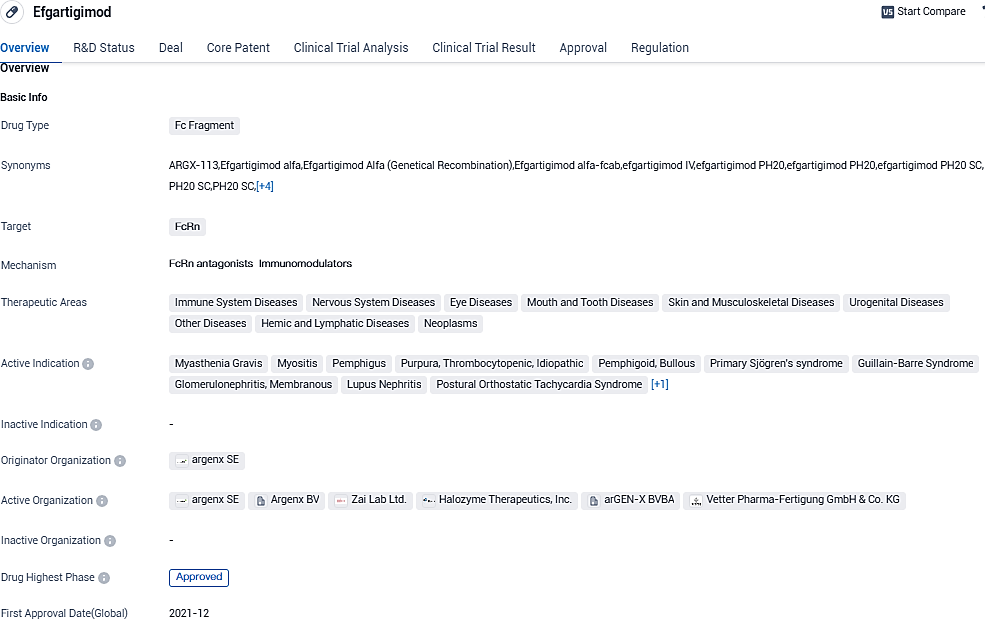
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In August 2022, VYVGART IV was initially greenlit by the EC. Post the endorsement, VYVGART has gained approval across Europe for both IV and self-administered SC use. This sanction holds for all 27 European Union member countries, including Iceland, Norway, and Liechtenstein. argenx is dedicated to collaborating with regional health bodies to ensure patient reach for VYVGART SC in these areas.
"Our dedication to offering an assortment of effective, cutting-edge treatments for individuals with autoimmune disorders is evident in this approval. We are jubilant to bring this second formulation to the European gMG community, a little more than a year after VYVGART IV received its first approval," stated Anant Murthy, the EMEA General Manager of argenx.
Murthy further added, "Having two formulations at disposal, including the one that patients can self-administer at home, empowers individuals afflicted with gMG to opt for the therapy that aligns better with their lifestyle. This underscores the personalized treatment strategy brought forward by VYVGART."
The approval from EC came on the heels of a favorable suggestion from the Committee for Medicinal Products for Human Use, and it pivots on the encouraging outcomes from the Phase 3 ADAPT-SC study. The efficacy of VYVGART SC was proven in the ADAPT- SC trial by showcasing a decrease in anti-AChR antibody concentrations on par with those of VYVGART IV in adult gMG patients. The ADAPT-SC served as a connective study to the Phase 3 ADAPT research, which laid the foundation for VYVGART IV's approval in Europe in August 2022.
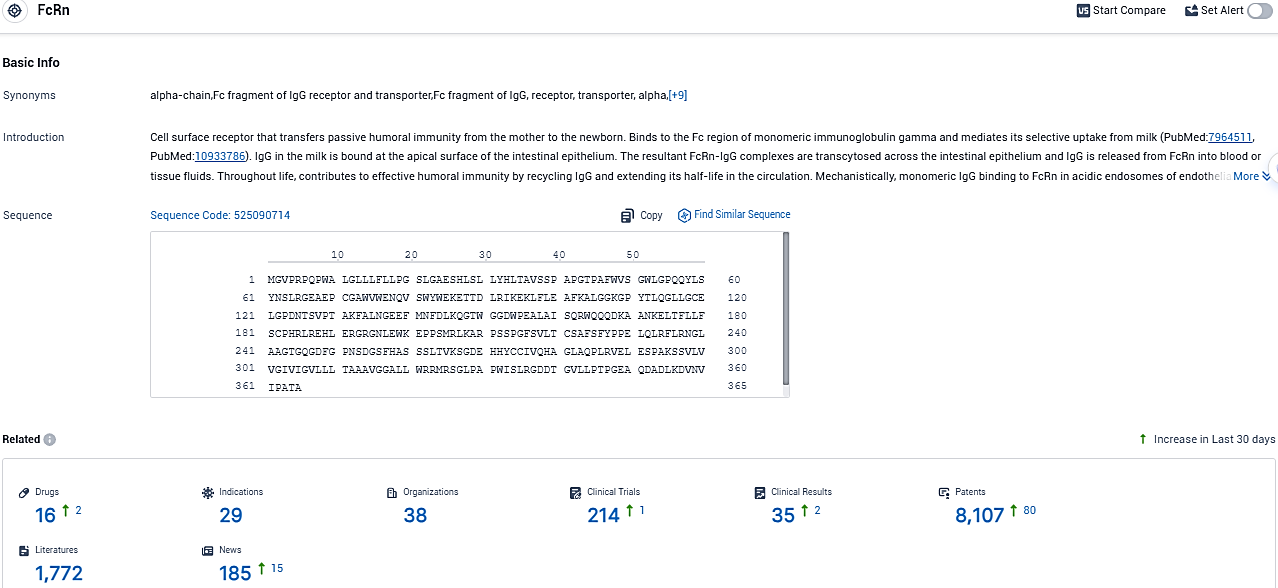
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 18, 2023, there are 16 investigational drugs for the FcRn target, including 29 indications, 38 R&D institutions involved, with related clinical trials reaching 214, and as many as 8107 patents.
VYVGART SC is a SC injectable formulation of efgartigimod alfa, a human IgG1 antibody fragment marketed for intravenous use as VYVGART. It is formulated with recombinant human hyaluronidase PH20, Halozyme’s ENHANZE® drug delivery technology, to facilitate SC injection delivery of biologics. In binding to the neonatal Fc receptor (FcRn), VYVGART results in the reduction of circulating IgG autoantibodies. VYVGART SC was approved in the United States in June 2023 and is marketed as VYVGART® Hytrulo.