Exploring KT-333's R&D successes and its clinical results at the 2023 ASH
The latest clinical findings of KT-333 will be unveiled at the 2023 ASH Congress, setting the stage for subsequent investigations.
KT-333's R&D Progress
KT-333 is a drug classified as PROTACs, which stands for Proteolysis Targeting Chimeras. This type of drug works by harnessing the body's own protein degradation system to selectively remove disease-causing proteins. The specific target of KT-333 is STAT3, a protein that plays a crucial role in various cellular processes and has been implicated in the development and progression of several diseases. The therapeutic areas in which KT-333 is being investigated include neoplasms (abnormal growth of cells), immune system diseases, and hemic and lymphatic diseases. 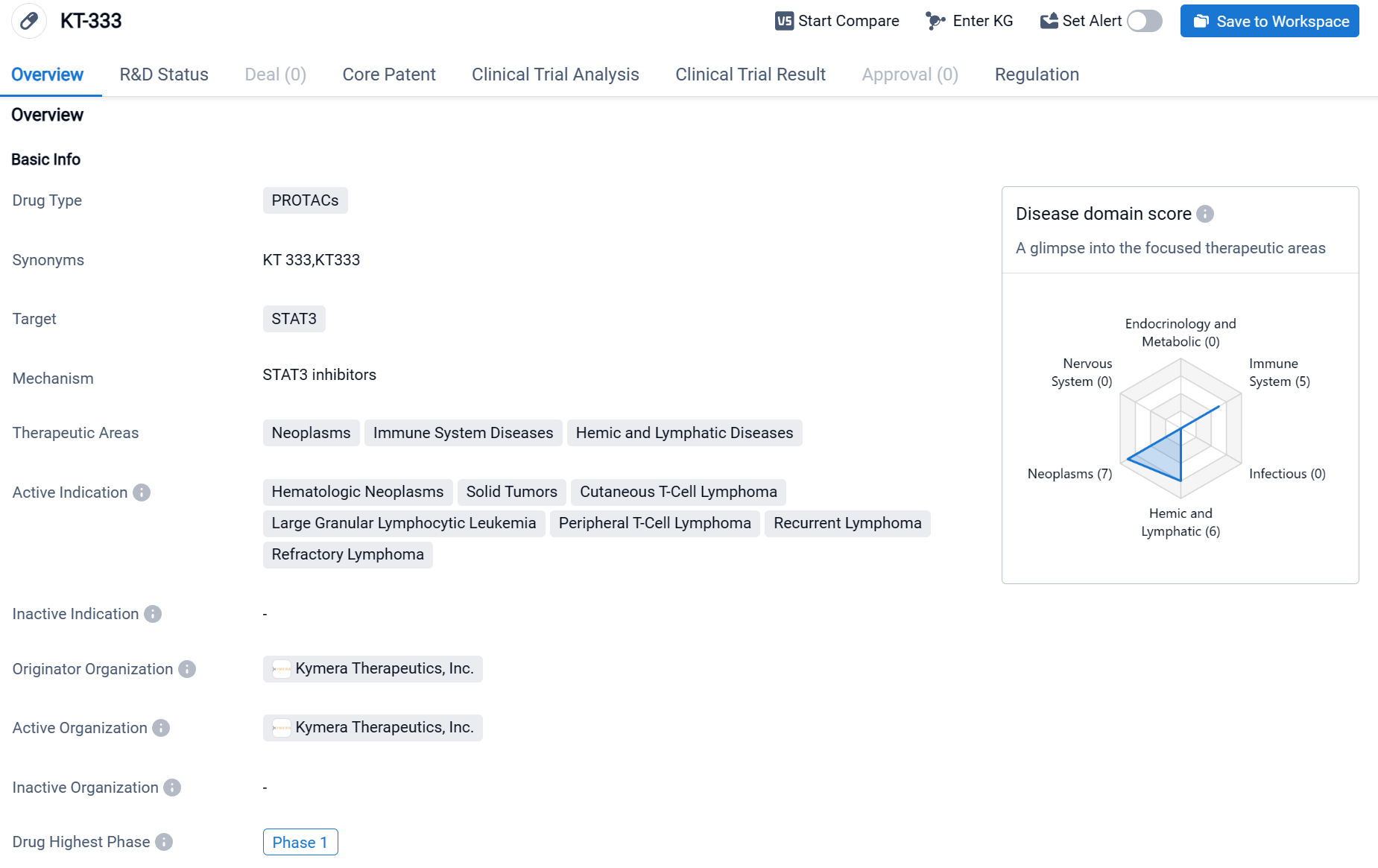
According to the Patsnap Synapse, KT-333 has reached the highest phase of clinical development, which is Phase 1. And the clinical trial area for KT-333 is primarily in the United States. The key indication is Peripheral T-Cell Lymphoma. 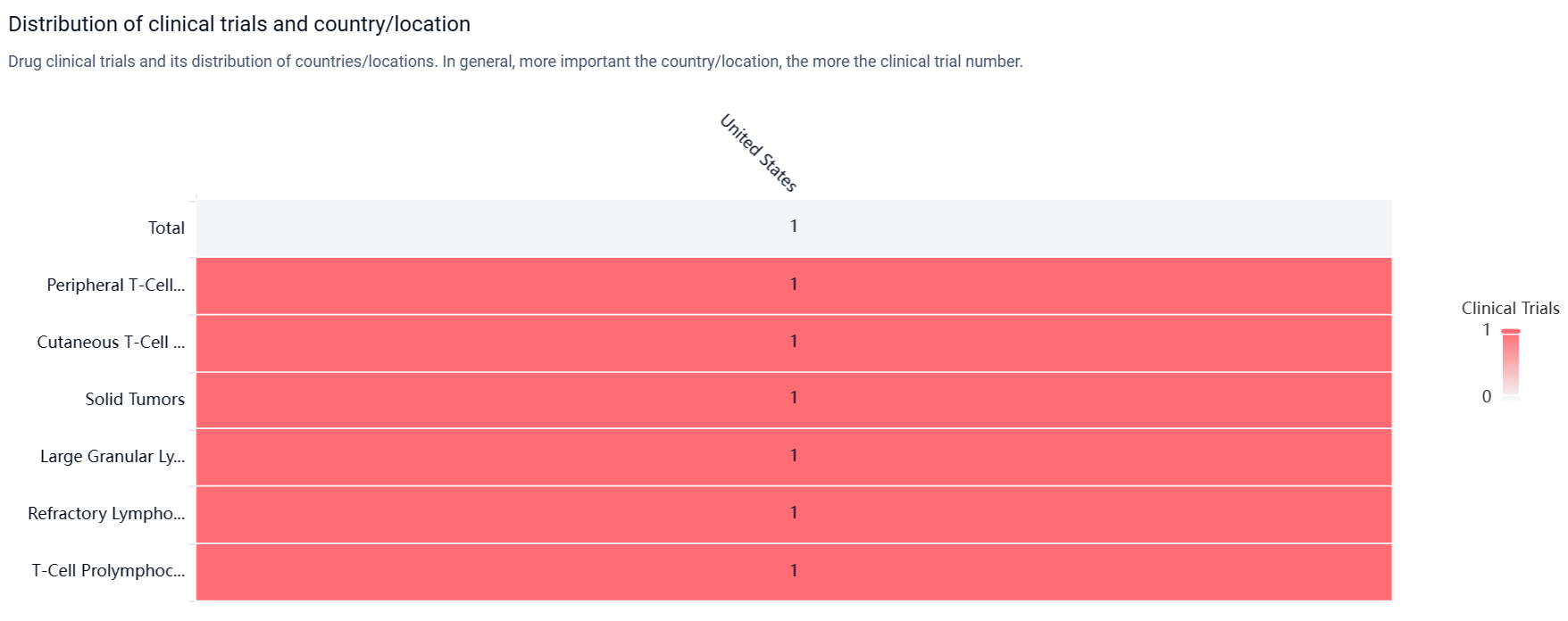
Detailed Clinical Result of KT-333
This ongoing open-label, Phase 1a/1b study is evaluating the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and preliminary clinical activity of KT-333 administered as a QW IV infusion on Day 1, 8, 15 and 22 (28-day cycle) in patients (pts) with B- and T-cell lymphomas, Hodgkin’s lymphoma and advanced solid tumors (ST) relapsed/refractory (R/R) to at least two prior systemic therapies and LGL-L and T-cell prolymphocytic leukemia R/R to at least one prior therapy.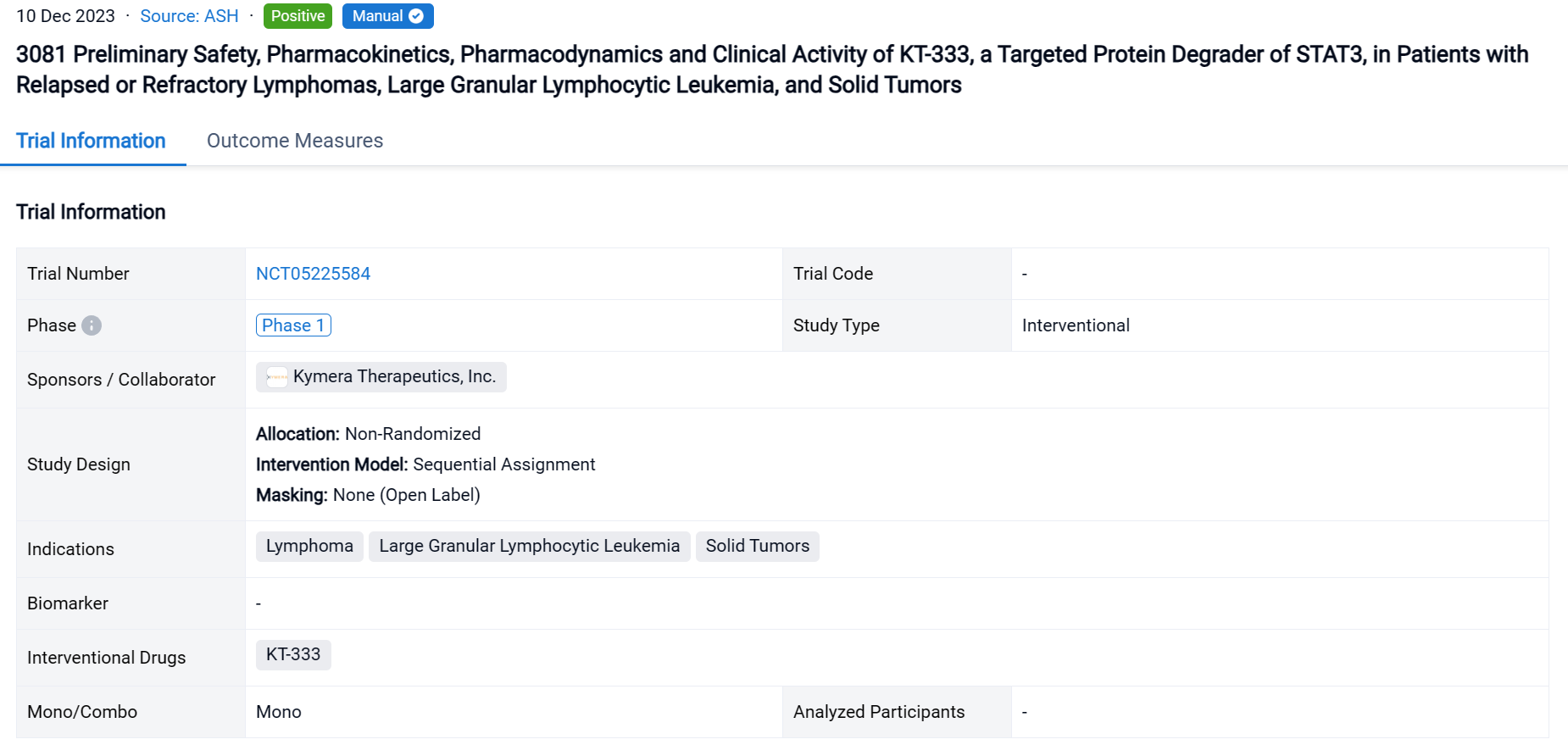
In this study, cycle 1 and 2 blood samples are collected for KT-333 plasma concentrations and to measure changes in STAT3 protein expression in peripheral blood mononuclear cells (PBMCs) using targeted mass spectrometry. Whole blood RNA sequencing measures mRNA levels of STAT3 regulated targets. Plasma levels of inflammatory biomarkers are measured with Luminex. STAT3 degradation and other related biomarker changes in tumor are assessed in patients with accessible tumors.
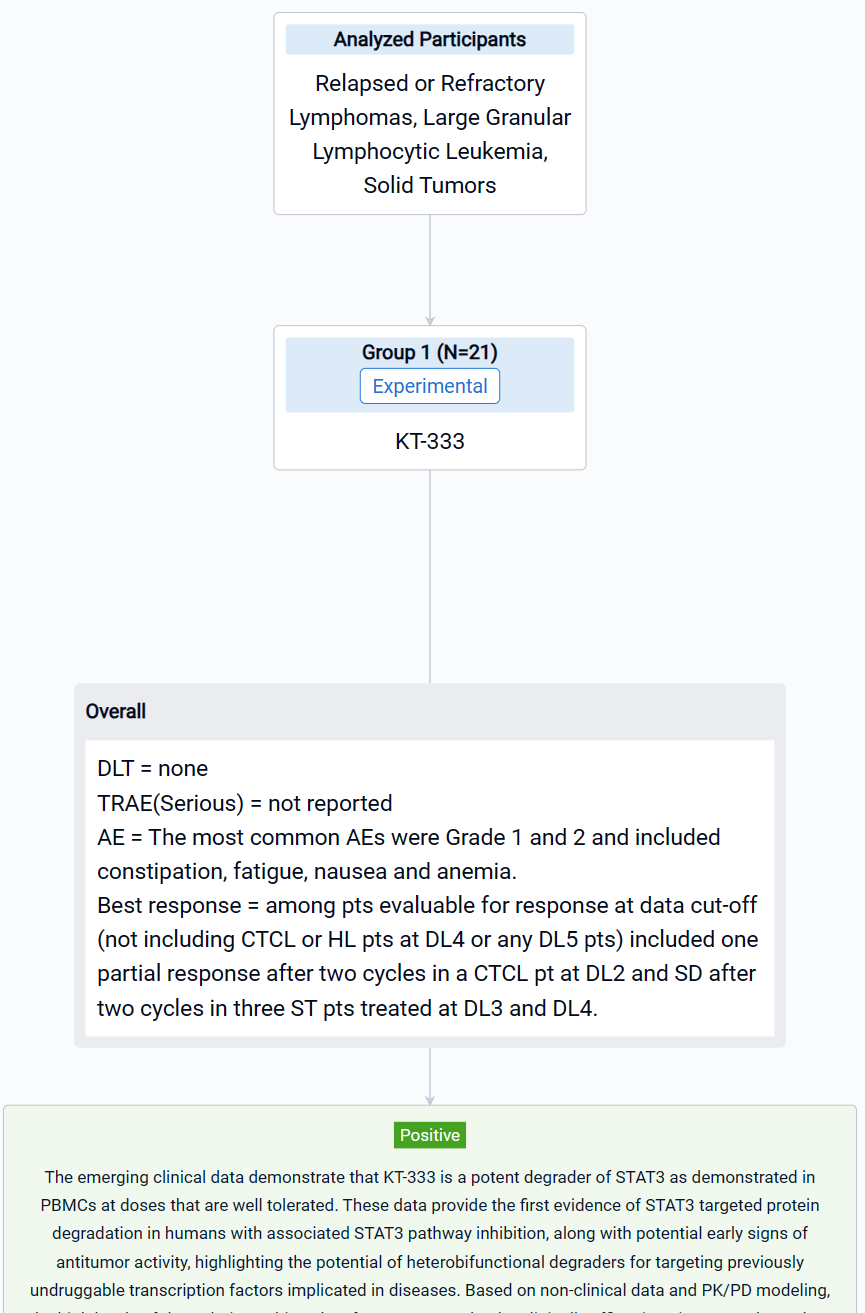
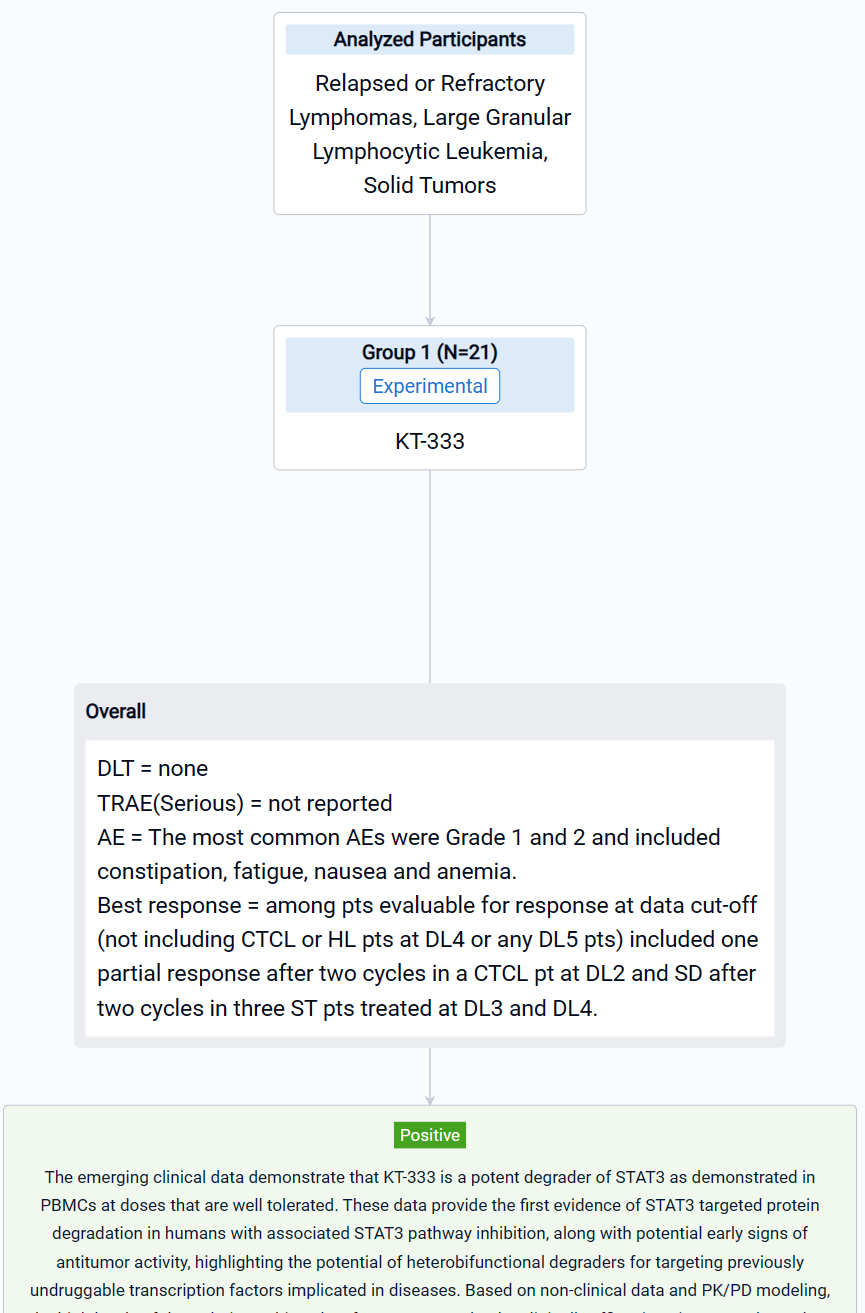
The result showed that as of July 10, 2023, 21 pts were treated at five dose levels (DL) in Phase 1a with a mean number of 5.8 doses. Pts included B-cell non-Hodgkin’s lymphoma (n=1: DL5), Hodgkin’s lymphoma (HL) (n=1: DL4), CTCL (n=3: DL1, 2 and 4), PTCL (n=1: DL2), LGL-L (n=2: DL5) and ST (n=13: DL1-4) with median age of 61 years (range 30,77) and ECOG performance status of 0 (n=7) or 1 (n=14). No DLTs and no KT-333 related serious adverse events (SAE) were reported. The most common AEs were Grade 1 and 2 and included constipation, fatigue, nausea and anemia. Best response among pts evaluable for response at data cut-off (not including CTCL or HL pts at DL4 or any DL5 pts) included one partial response after two cycles in a CTCL pt at DL2 and SD after two cycles in three ST pts treated at DL3 and DL4. PD data in blood available for DL1-4 demonstrated robust, dose-dependent, and sustained STAT3 degradation in PBMC. The mean maximum degradation of STAT3 by targeted mass spectrometry over the first two weeks in Cycle 1 by DL was (% (range; n)): DL1: 69.9% (52.6% to 84.1%; n=4), DL2: 73.5% (65.5% to 80.7%; n=3), DL3: 79.9% (72.3% to 90.4 %; n=3) and DL4: 86.6% (78.9% to 95.9 %; n=4) with absolute quantification of STAT3 peptides falling below lower limit of quantification of the assay for one pt in DL3 and two in DL4. STAT3 pathway inhibition in blood was demonstrated via transcriptional downregulation of a canonical JAK/STAT3 target, SOCS3, which correlated with changes in STAT3 protein levels. KT-333 also resulted in dose-dependent downregulation of STAT3-regulated inflammatory biomarkers C-reactive protein and serum amyloid A protein in plasma. KT-333 demonstrated linear PK with plasma exposure increasing with dose and reaching levels close to those predicted to be efficacious.
It can be concluded that KT-333 is a potent degrader of STAT3 as demonstrated in PBMCs at doses that are well tolerated. These data provide the first evidence of STAT3 targeted protein degradation in humans with associated STAT3 pathway inhibition, along with potential early signs of antitumor activity, highlighting the potential of heterobifunctional degraders for targeting previously undruggable transcription factors implicated in diseases. Based on non-clinical data and PK/PD modeling, the high levels of degradation achieved so far are expected to be clinically efficacious in STAT3-dependent malignancies. Accrual is ongoing, and analyses from additional patients will be presented at the meeting.
How to Easily View the Clinical Results Using Synapse Database?
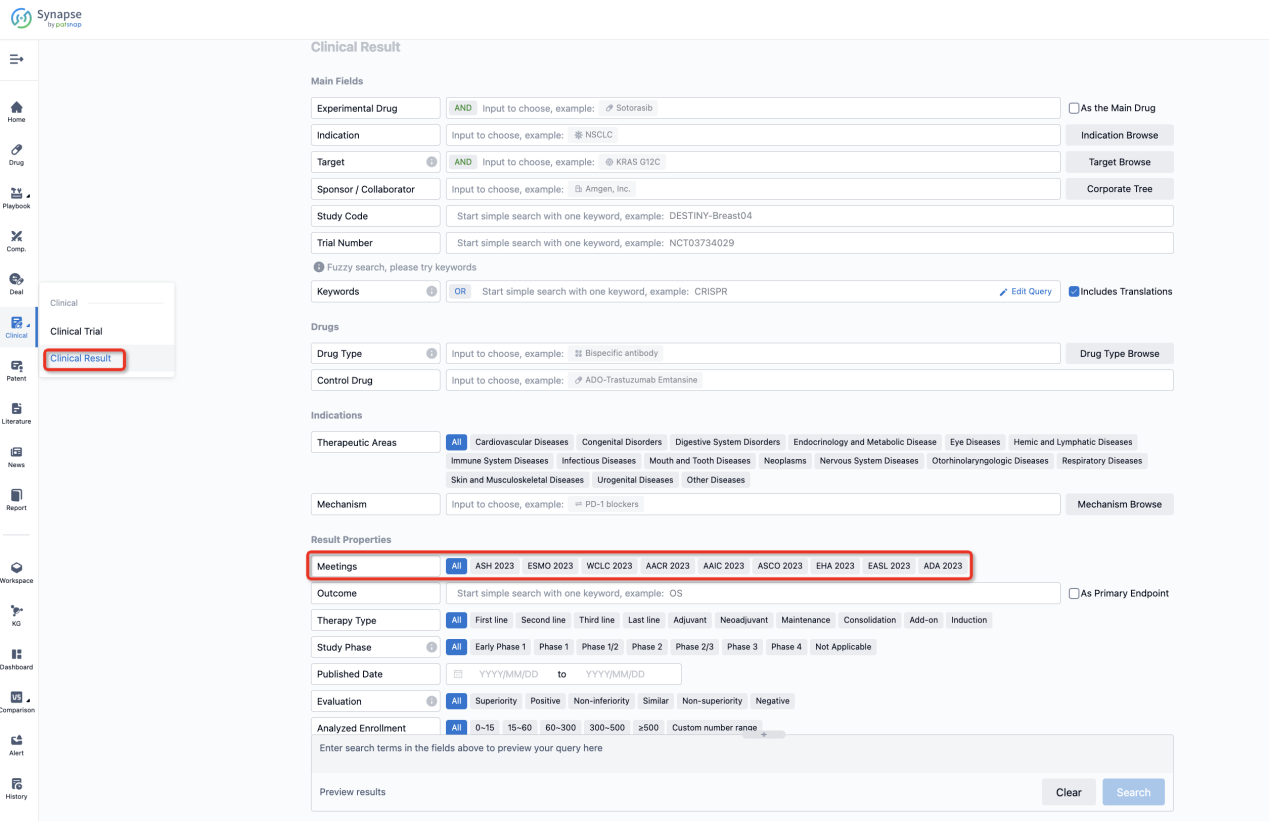
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
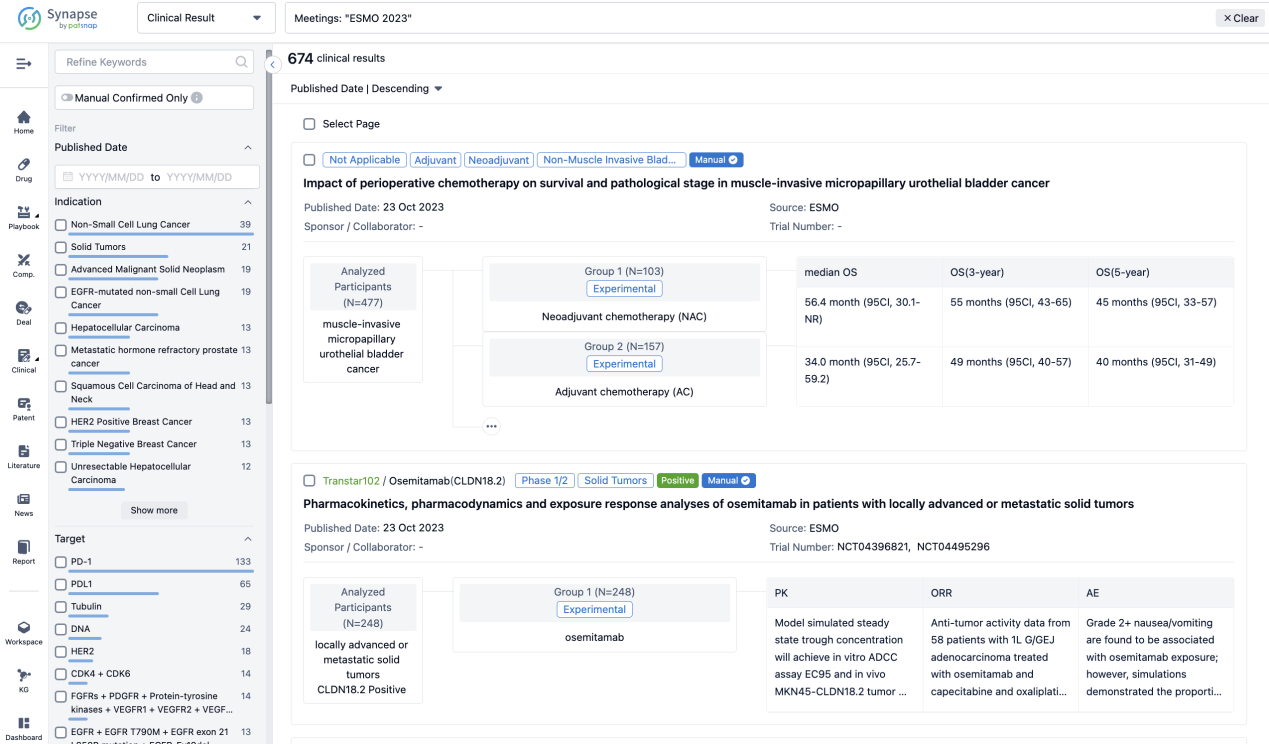
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

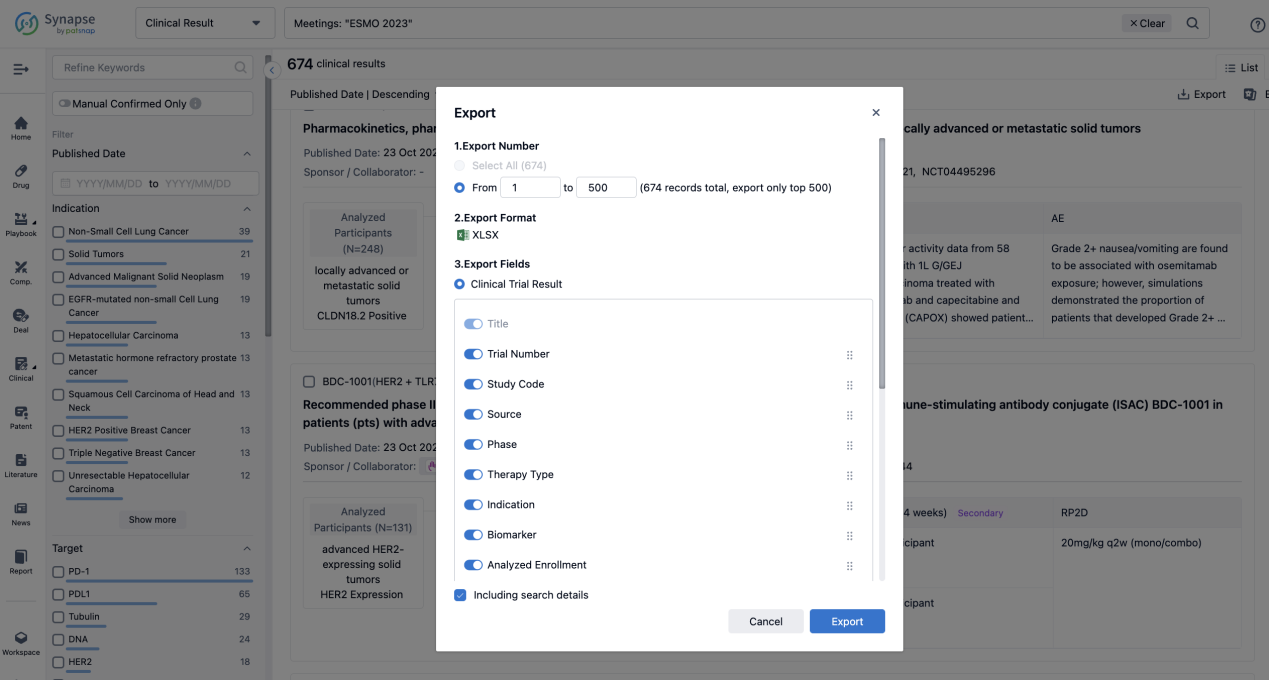
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!