Arrowhead Pharmaceuticals has applied to start a phase 1 trial for ARO-DM1 in Myotonic Dystrophy Type 1 patients
Arrowhead Pharmaceuticals Inc. has disclosed its submission of regulatory paperwork seeking authorization to commence a Stage 1/2a clinical study for ARO-DM1. This experimental RNAi-based therapeutic is being crafted by the company with the aim to address type 1 myotonic dystrophy, which stands as the predominant form of muscular dystrophy affecting adults.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
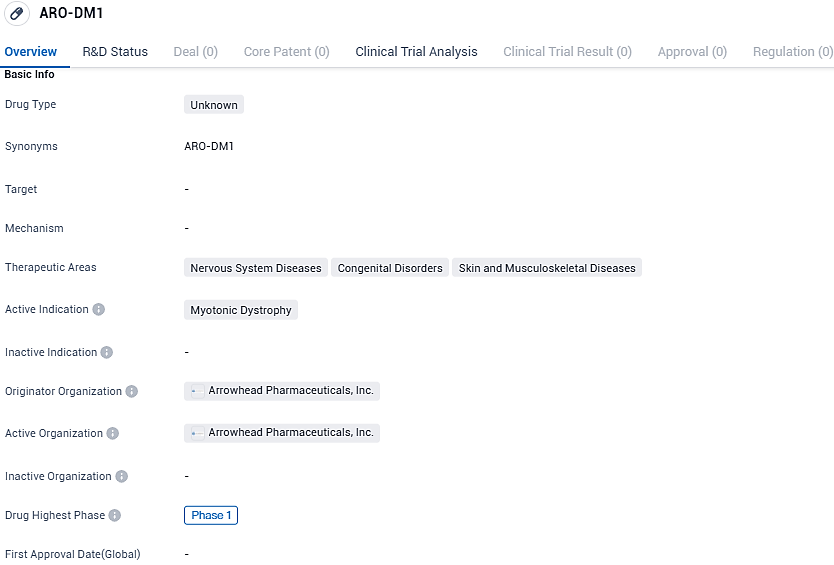
ARO-DM1 has been developed to mitigate the production of the dystrophia myotonica protein kinase gene. Currently, there is an absence of certified treatment options that alter the progression of DM1. Existing therapeutic measures are directed towards alleviating symptoms through options like rehabilitative exercises, occupational therapy, support braces for the ankle and foot, mobility aids like wheelchairs, and various supportive technologies.
Constructed by Arrowhead, ARO-DM1 is the pharmaceutical company's second investigational drug conceived under the proprietary TRiM™ technology that targets RNA interference mechanisms within skeletal muscle tissue. Individuals suffering from DM1 typically experience progressive muscular degradation and weakness, myotonia, ocular lens clouding that may lead to cataracts, and risk developing complications related to heart rhythm which may result in both physical incapacitation and reduction of life expectancy.
The strategy behind ARO-DM1 involves the therapeutic silencing of the erroneously expressed DMPK mRNA, with the potential to alleviate a host of problematic symptoms like compromised muscle functionality and endurance.
In pursuit of clinical validation, the proposal for the clinical investigation of ARO-DM1 underwent submission to the local ethical regulatory body and the Medicines and Medical Devices regulator in New Zealand, specifically to the division responsible for evaluating therapeutic experiments. Should the authorization be granted, Arrowhead is prepared to launch the ARODM1-1001 clinical trial. This early-stage clinical study, categorized as Phase 1/2a, is designed as an ascending dose experiment to determine ARO-DM1’s safety profile, acceptability, kinetic interaction within the body, and its biological effect on up to 48 participants with DM1.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
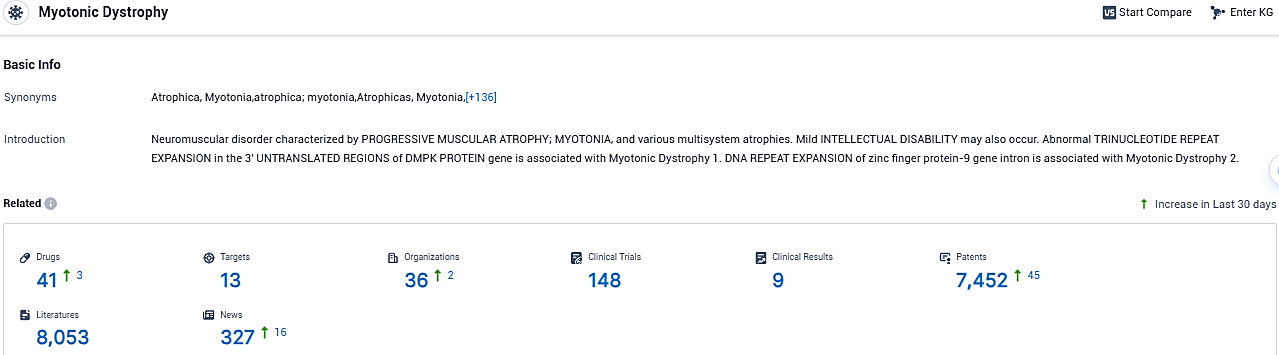
According to the data provided by the Synapse Database, As of December 6, 2023, there are 41 investigational drugs for Myotonic Dystrophy, including 13 targets, 36 R&D institutions involved, with related clinical trials reaching 148, and as many as 7452 patents.
ARO-DM1 developed for the treatment of Myotonic Dystrophy. Its therapeutic areas include nervous system diseases, congenital disorders, and skin and musculoskeletal diseases. Currently in Phase 1 of development, further research and clinical trials are needed to assess its potential benefits.