Arrowhead Pharmaceuticals Submits FDA Application for Familial Chylomicronemia Treatment
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) has revealed that it has filed a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) for its investigational drug, plozasiran, aimed at treating familial chylomicronemia syndrome (FCS), a rare and serious genetic condition that lacks any existing FDA-approved treatments. Additionally, Arrowhead plans to seek approval for plozasiran from other regulatory agencies in 2025 for FCS patients.
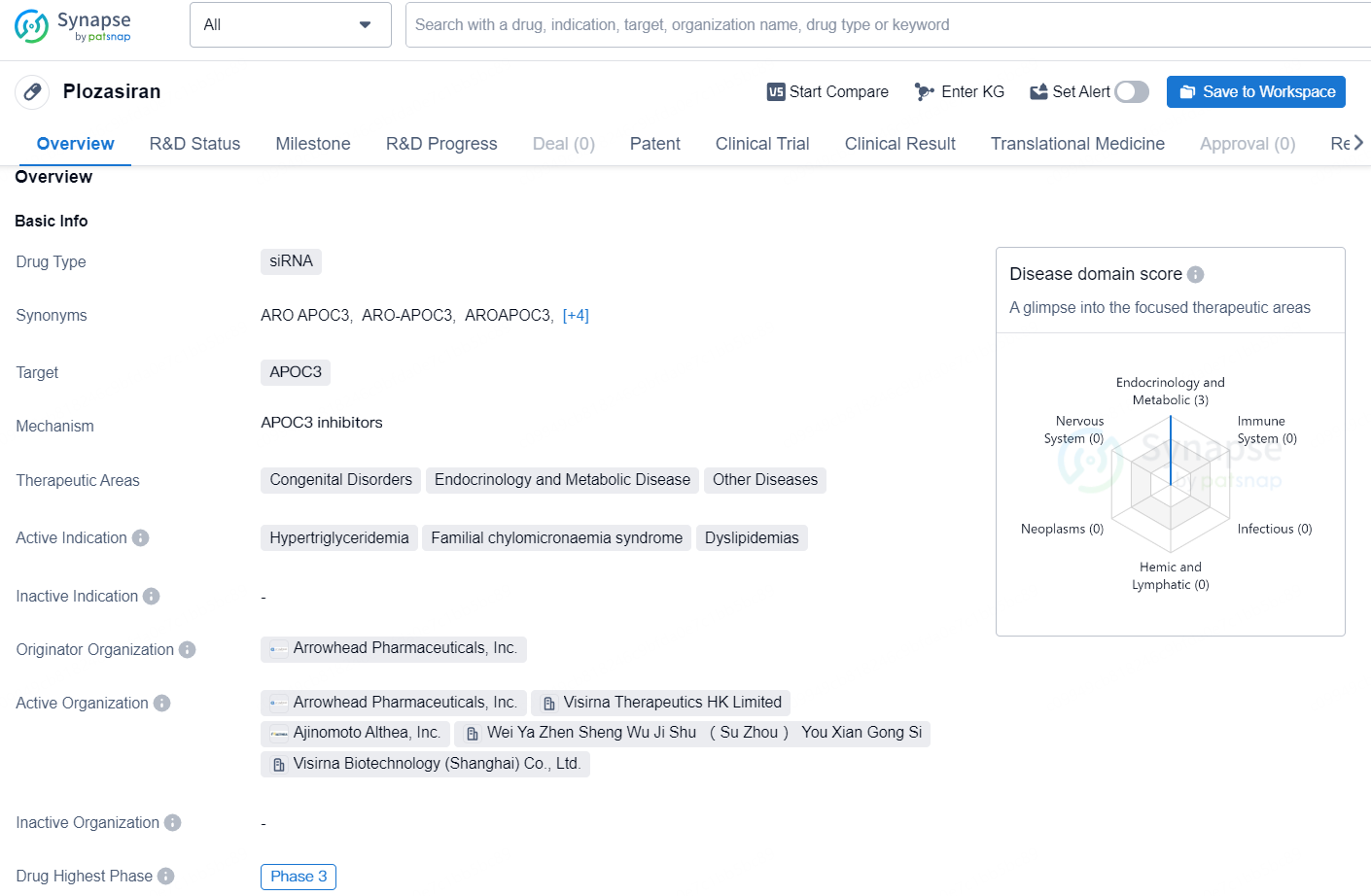
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The submission of the NDA for the investigational drug plozasiran marks a significant achievement for Arrowhead as we progress with several promising new therapies derived from our proprietary Targeted RNAi Molecule (TRiMTM) platform, aimed at addressing patients' needs across various therapeutic domains such as cardiometabolic, pulmonary, neuromuscular, and liver ailments,” stated Chris Anzalone, Ph.D., Arrowhead's President and CEO. "We are confident in RNAi's potential to significantly benefit patients, and this NDA submission represents the result of more than 15 years of dedication from numerous skilled Arrowhead team members, investigators, patients, and caregivers who brought our vision to fruition."
Bruce Given, M.D., Arrowhead's chief medical scientist, remarked, "The SUMMIT clinical trial initiative for plozasiran has yielded encouraging and consistent outcomes across diverse patient groups reflecting various levels of elevated triglycerides. Familial chylomicronemia syndrome (FCS) indicates the most critical end of this range, where many patients endure a severely diminished quality of life and are at heightened risk for acute pancreatitis, a potentially life-threatening condition. There are currently no FDA-approved treatments for FCS, so we are diligently working to expedite access to plozasiran for patients, subject to FDA evaluation and approval."
The NDA submission is bolstered by the results from the SUMMIT program of clinical trials for plozasiran and the positive outcomes observed in the Phase 3 PALISADE study. PALISADE successfully reached its primary goal and all key secondary objectives with statistical significance, including notable reductions in triglycerides (TGs), apolipoprotein C-III (APOC3), and occurrences of acute pancreatitis (AP).
In the PALISADE trial, plozasiran demonstrated substantial and sustained decreases in triglyceride levels, with a median reduction from baseline of 80% in the 25 mg plozasiran group and an statistically significant 83% decrease in the likelihood of developing acute pancreatitis compared to placebo, when considering both the 25 mg and 50 mg groups combined. Overall, plozasiran has been generally well-tolerated thus far. In the PALISADE study, the most commonly reported treatment-emergent adverse events for the 25 mg dose under consideration for marketing approval included abdominal discomfort, COVID-19, nasopharyngitis, and nausea.
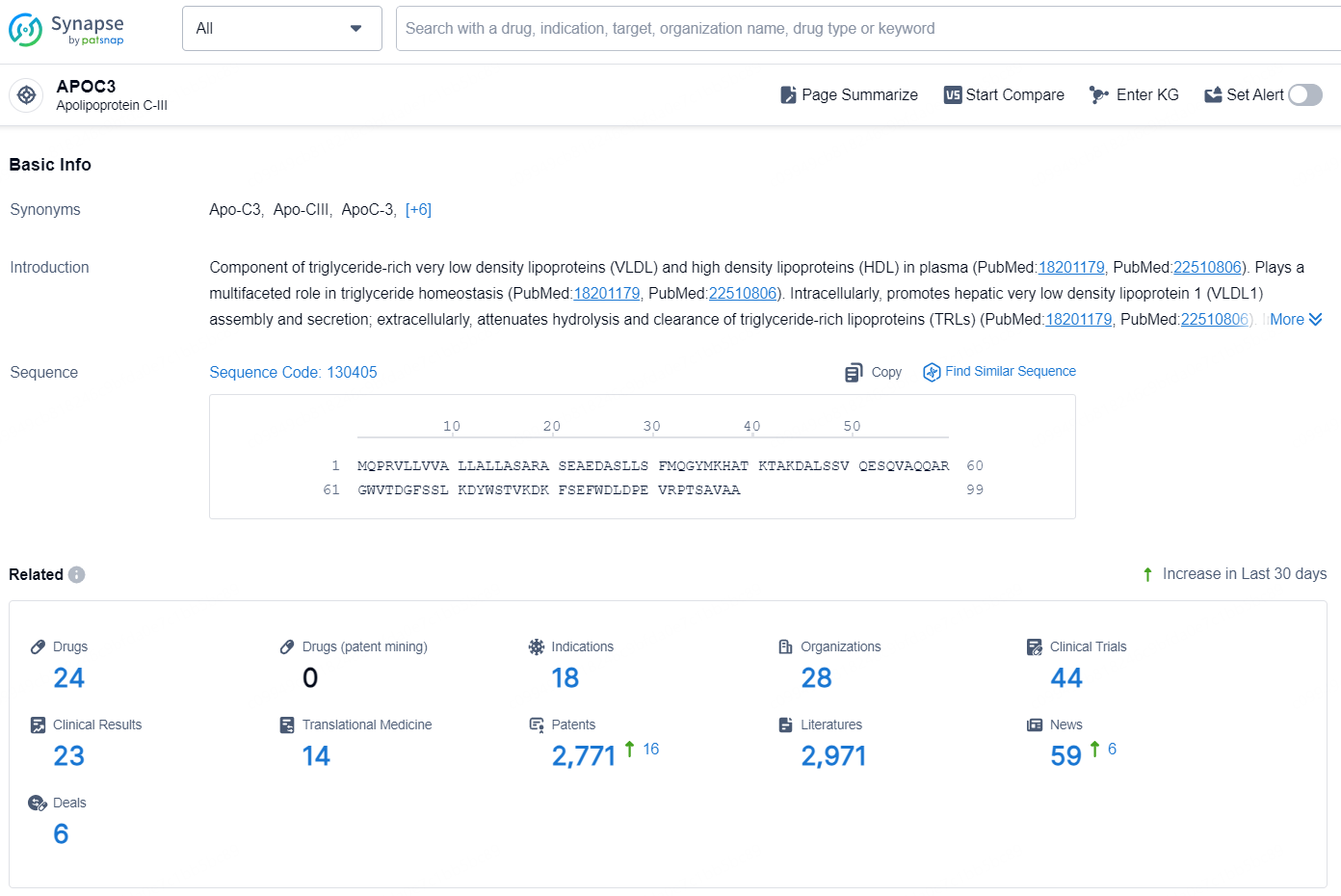
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 24 investigational drug for the APOC3 target, including 18 indications, 28 R&D institutions involved, with related clinical trials reaching 44, and as many as 2771 patents.
Plozasiran is a drug type siRNA that is designed to target APOC3, a protein associated with lipid regulation. It is being developed by Arrowhead Pharmaceuticals, Inc. The drug is aimed at addressing various therapeutic areas including Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases, with a specific focus on treating Hypertriglyceridemia, Familial chylomicronaemia syndrome, and Dyslipidemias.