Arrowhead Pharmaceuticals to Start Clinical Trials for Two New RNAi Obesity Drugs, ARO-INHBE and ARO-ALK7
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) disclosed preclinical data and outlined its strategy to move two advanced RNAi-based candidates, ARO-INHBE and ARO-ALK7, into forthcoming clinical trials for obesity and metabolic disorders. These candidates have shown promise in preclinical studies to date, demonstrating a novel mechanism of action that has the potential to decrease body weight and fat mass while potentially better preserving lean muscle mass compared to existing obesity therapies. Arrowhead aims to submit clinical trial applications to regulatory authorities for both candidates by the end of 2024, with plans to commence clinical trials in obese volunteers in early 2025.
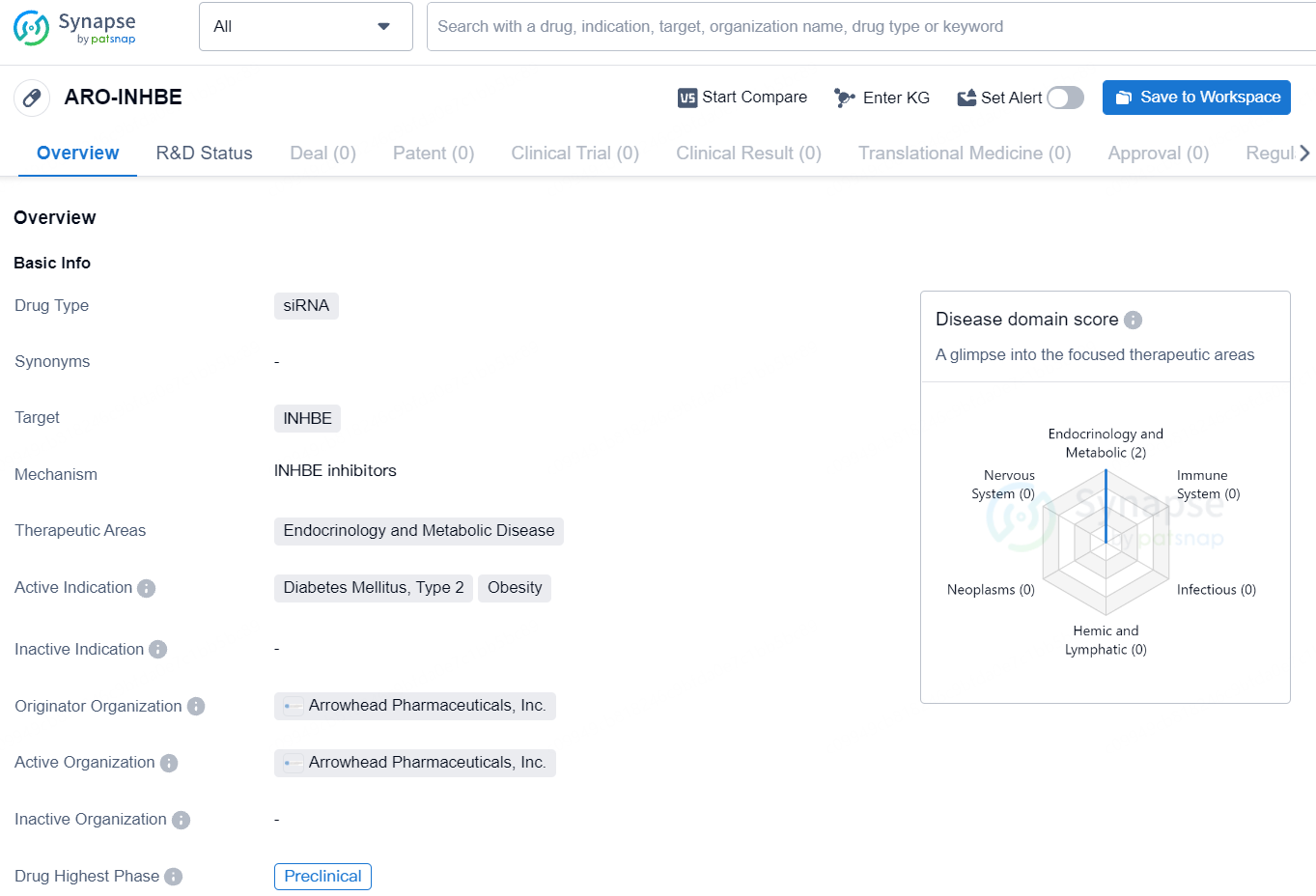
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Arrowhead posits that focusing on reducing the hepatic expression of the INHBE gene and Activin E ligand production, along with the adipose tissue expression of the ALK7 gene and its corresponding receptor, presents novel and promising mechanisms for addressing obesity and related metabolic disorders. In studies using diet-induced obesity mouse models, both as standalone treatments and in combination with tirzepatide, ARO-INHBE and ARO-ALK7 led to a decrease in body weight and fat mass while maintaining lean mass, thereby enhancing overall body composition, according to James Hamilton, M.D., Chief of Discovery and Translational Medicine at Arrowhead. "Given the recent successes and clinical advancements in obesity treatments, innovative therapeutic approaches with new mechanisms of action are emerging, potentially shaping the future of obesity and associated disease management. We are nearing the end of preclinical development for ARO-INHBE and ARO-ALK7 and anticipate engaging with regulators later this year to initiate clinical trials for these exciting new therapies."
ARO-INHBE aims to reduce hepatic INHBE gene expression and its protein product, Activin E. ARO-ALK7 targets the reduction of ALK7, or Activin receptor-like kinase 7, expression in adipose tissue. Both INHBE and ALK7 are validated genetic targets, with loss-of-function variants linked to a reduced risk of obesity and metabolic disorders such as type 2 diabetes. Both targets are implicated in a pathway that governs energy homeostasis in adipose tissue, where Activin E functions as a circulating ligand and ALK7 serves as a receptor on adipocytes. Intervention in this pathway using ARO-INHBE and ARO-ALK7 is believed to enhance lipolysis, diminish adipose hypertrophy and dysfunction, decrease visceral fat, and improve insulin sensitivity.
For more information on ARO-INHBE and ARO-ALK7, please attend Part IV of Arrowhead’s 2024 Summer Series of R&D Webinars today, August 14, 2024, at 2:00 PM ET. The session will feature presentations from Arrowhead management and Carel le Roux, M.D., Ph.D., from University College Dublin. The live event and an archived webcast can be accessed via the Events and Presentations page in the Investors section of the Arrowhead website.
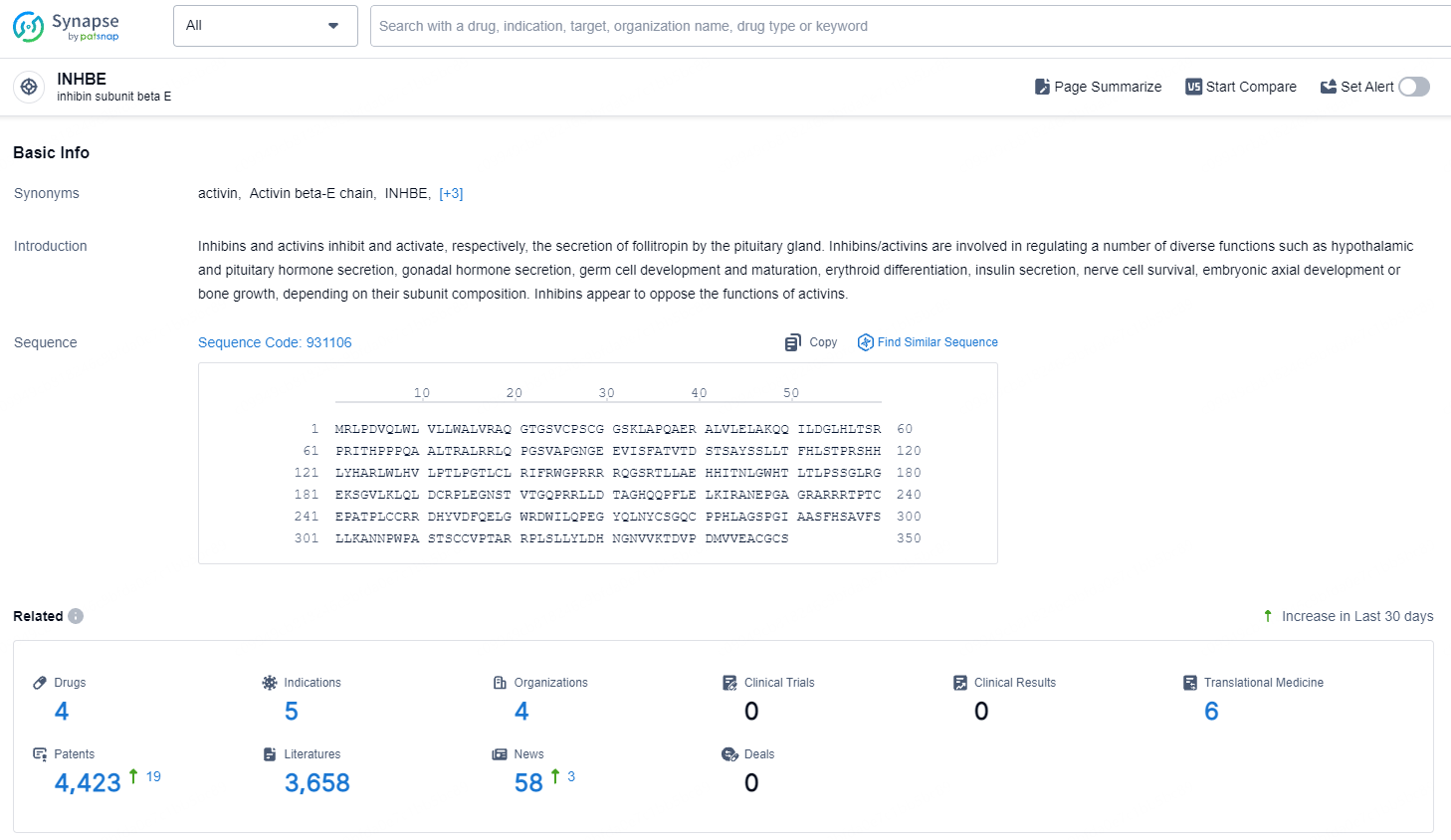
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 19, 2024, there are 4 investigational drugs for the INHBE target, including 5 indications, 4 R&D institutions involved, and as many as 4423 patents.
The drug ARO-INHBE is a siRNA-based therapeutic targeting INHBE, developed by Arrowhead Pharmaceuticals, Inc. It falls under the therapeutic areas of Endocrinology and Metabolic Disease, with a focus on treating Diabetes Mellitus, Type 2, and Obesity. As a small interfering RNA (siRNA) drug, ARO-INHBE works by targeting the INHBE gene to inhibit its expression, which can potentially have a therapeutic effect on the indicated conditions. siRNA drugs are designed to interfere with the production of specific proteins, offering a promising approach for treating a variety of diseases at the genetic level.