Artemether: Detailed Review of its Transformative R&D Success
Artemether's R&D Progress
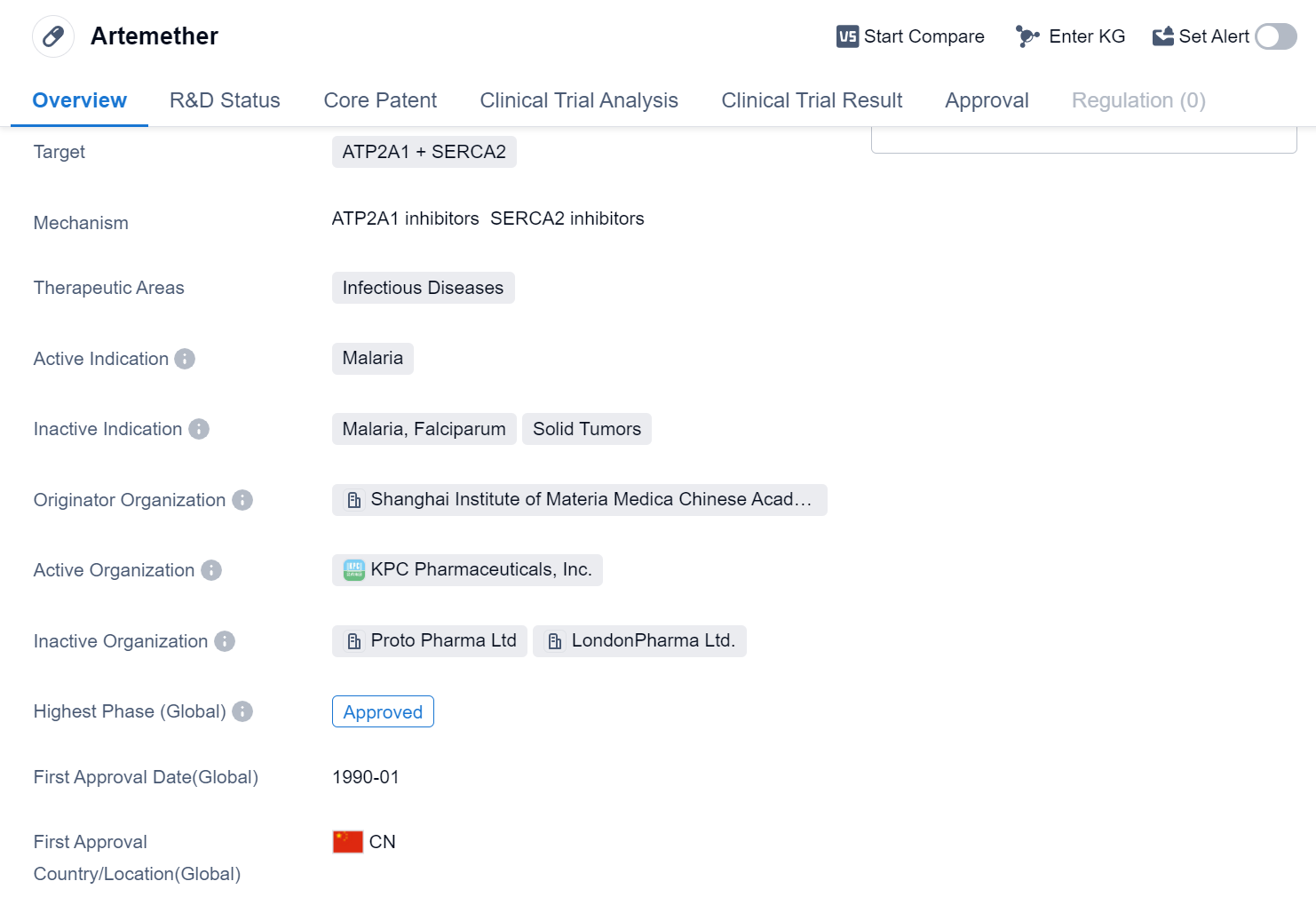
Artemether is a small molecule drug that falls under the therapeutic area of infectious diseases, specifically targeting ATP2A1 and SERCA2. It is primarily used for the treatment of malaria. The drug was developed by the Shanghai Institute of Materia Medica, which is a part of the Chinese Academy of Sciences.
Artemether has been approved for use in the global market. Its highest R&D phase is approved. The drug received its first approval in China in January 1990.
As a small molecule drug, Artemether is designed to interact with specific targets in the body, in this case, ATP2A1 and SERCA2. These targets are believed to play a crucial role in the treatment of malaria. By targeting these specific proteins, Artemether aims to disrupt the life cycle of the malaria parasite, ultimately leading to its elimination from the body.
The Shanghai Institute of Materia Medica, as the originator organization of Artemether, has played a significant role in its development and subsequent approvals. The Chinese Academy of Sciences, under which the institute operates, is a prestigious research institution known for its contributions to various scientific fields.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Artemether: ATP2A1 inhibitors & SERCA2 inhibitors
ATP2A1 inhibitors, also known as SERCA2 inhibitors, are a type of drugs that target and inhibit the ATP2A1 protein, which is also known as the sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2).
From a biomedical perspective, SERCA2 is an important protein found in the sarcoplasmic reticulum of muscle cells. It plays a crucial role in regulating calcium levels within the cell. By inhibiting ATP2A1/SERCA2, these inhibitors disrupt the normal calcium cycling process in muscle cells.
In general understanding, ATP2A1 inhibitors or SERCA2 inhibitors are drugs that interfere with the function of the ATP2A1 protein, leading to altered calcium regulation within cells. This disruption can have various effects depending on the specific context, but in the case of SERCA2 inhibitors, it primarily affects muscle cells.
It's worth noting that ATP2A1 inhibitors or SERCA2 inhibitors can have potential therapeutic applications in conditions where abnormal calcium handling is involved, such as heart failure, cardiac arrhythmias, and certain skeletal muscle disorders. By modulating calcium levels, these inhibitors may help restore normal cellular function and improve overall health outcomes.
Drug Target R&D Trends for Artemether
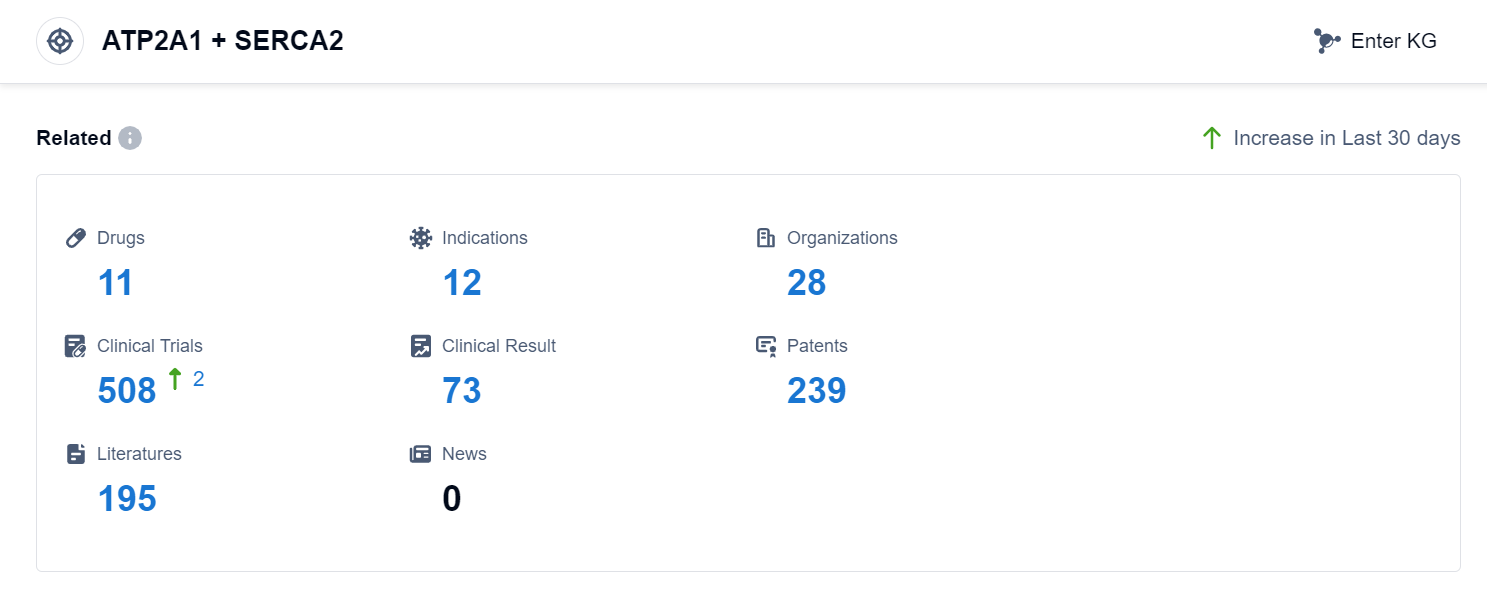
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 11 ATP2A1 + SERCA2 drugs worldwide, from 28 organizations, covering 12 indications, and conducting 508 clinical trials.
The analysis of the target ATP2A1 + SERCA2 reveals a competitive landscape with multiple companies actively involved in the development of drugs. Novartis AG, Shinpoong Pharmaceutical Co., Ltd., and China Resources Pharmaceutical Group Ltd. are the companies growing fastest under this target. The approved drugs target indications such as Malaria, COVID-19, and various types of neoplasms. Small molecule drugs are progressing rapidly, indicating potential advancements in this area. China is a prominent player in the development of drugs targeting ATP2A1 + SERCA2. Overall, the target ATP2A1 + SERCA2 shows promise for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Artemether is a small molecule drug developed by the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. It is approved for the treatment of malaria and targets ATP2A1 and SERCA2. With its first approval in China in 1990, Artemether has been widely used for several decades in combating malaria.