Arvinas and Novartis Seal Global Licensing Deal for Prostate Cancer Therapy ARV-766
Arvinas, Inc., an enterprise operating at the clinical phase in the biotechnological sector, focused on pioneering a novel category of pharmaceuticals through the targeted protein degradation approach, has revealed its entry into a sole comprehensive licensing contract with the global healthcare giant Novartis. The agreement encompasses the international advancement and marketing rights for their investigational compound, ARV-766.
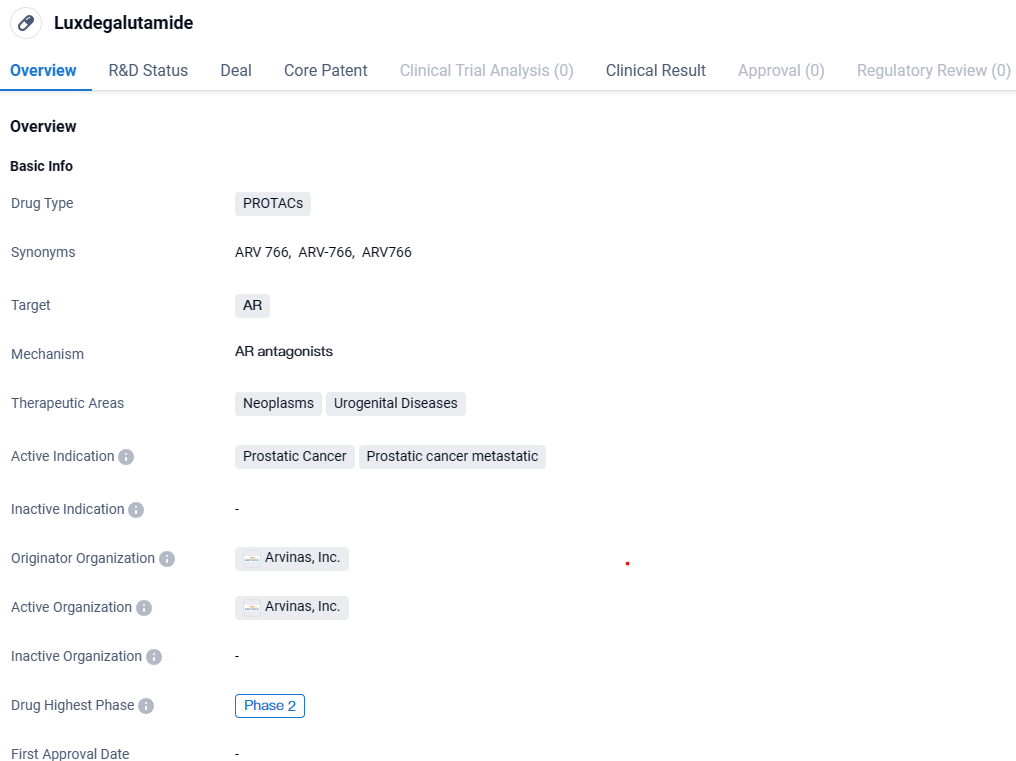
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Arvinas is advancing its progressive PROTAC technology by transferring its second-generation androgen receptor (AR) degrader designed to combat prostate cancer. This shift includes a deal where Arvinas will sell its AR-V7 program that's still in the early stages of development to Novartis.
"It's incredibly exciting to align with an entity that echoes our commitment to pioneering critical treatments for patients with grave health conditions," stated John Houston, Ph.D., the leader, president, and CEO at Arvinas. "Novartis' knowledge and resources are expected to enhance the progression of ARV-766, increasing its prospects of becoming a premier and unrivaled therapy for individuals facing prostate cancer. Our agreement further acknowledges the groundbreaking nature of our PROTAC technology in creating novel therapies."
As per the agreement's provisions, Novartis gains complete responsibility for global clinical trials, market introduction, and overall rights tied to research, development, production, and market launch related to the AR-V7 project in its early stages. Arvinas will initially obtain a payment totaling $150 million. Furthermore, according to the Licensing Agreement, Arvinas stands to gain additional payments linked to development milestones and regulatory and sales benchmarks, potentially accumulating in excess of $1.01 billion, along with staggered royalties for ARV-766.
The deal's finalization is contingent upon both parties obtaining requisite clearances or endorsements, which includes the lapse or conclusion of the review period as stipulated by the Hart-Scott-Rodino Antitrust Improvements Act of 1976. Goldman Sachs & Co. LLC serves as Arvinas' sole financial consultant for this transaction.
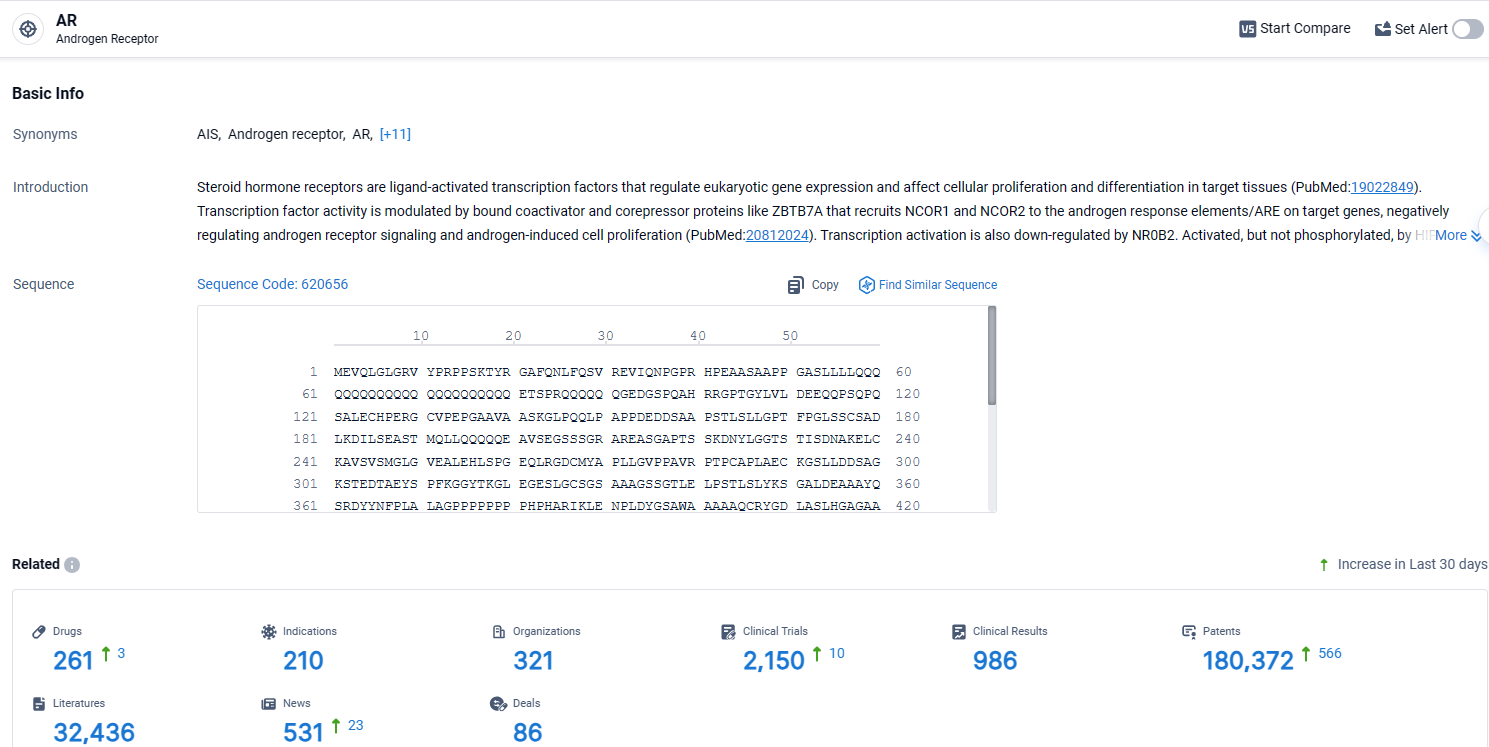
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 15, 2024, there are 261 investigational drugs for the AR target, including 210 indications, 321 R&D institutions involved, with related clinical trials reaching 2150, and as many as 180372 patents.
ARV-766 is a PROTACs drug that targets the androgen receptor and is primarily used for the treatment of prostatic cancer and metastatic prostatic cancer. It falls under the therapeutic areas of neoplasms and urogenital diseases. Luxdegalutamide has reached Phase 2 of clinical development, indicating promising progress in its evaluation as a potential treatment option.