Arvinas & Pfizer Present Phase 1b TACTIVE-U Results on Vepdegestrant + Abemaciclib at 2024 SABCS
Arvinas, Inc. (Nasdaq: ARVN) and Pfizer Inc. (NYSE: PFE) have revealed initial findings from the ongoing Phase 1b segment of the TACTIVE-U sub-study, which examines the use of vepdegestrant in conjunction with abemaciclib in patients diagnosed with locally advanced or metastatic estrogen receptor positive (ER+)/human epidermal growth factor receptor 2 negative (HER2-) breast cancer. These findings are set to be shared in a poster presentation at the 2024 San Antonio Breast Cancer Symposium (SABCS) taking place in San Antonio, Texas.
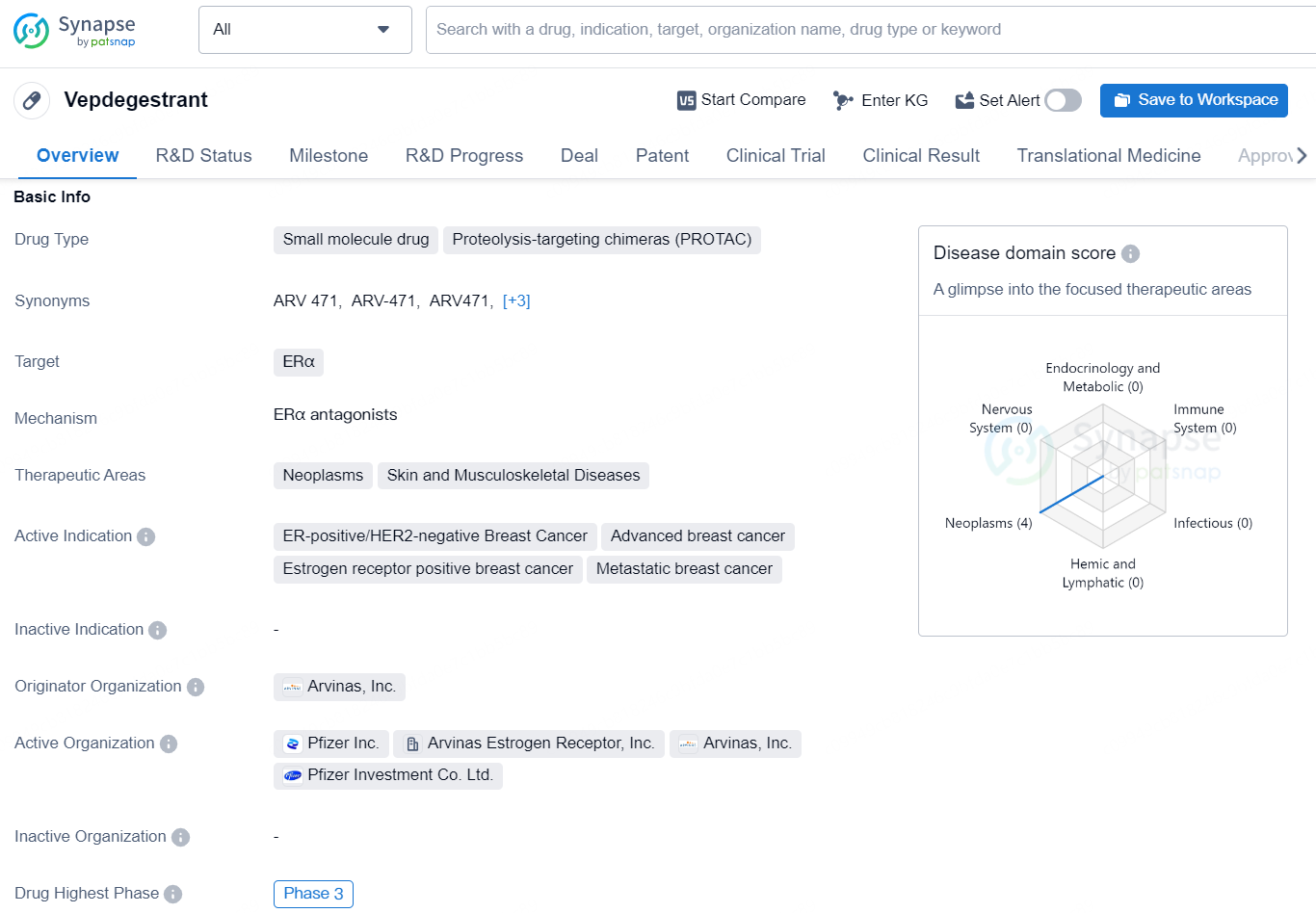
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Initial findings from a cohort of 16 patients in the Phase 1b sub-study indicated that the combination of abemaciclib (150 mg twice daily) and the recommended Phase 3 dose of vepdegestrant (200 mg once daily) exhibited an acceptable safety profile. A promising clinical benefit rate of 62.5% was recorded among patients harboring both mutant and wild-type ESR1 mutations, all of whom had undergone prior treatment with a CDK4/6 inhibitor.
The pharmacokinetic analysis revealed no considerable drug-drug interactions between vepdegestrant and abemaciclib, with no significant impact on the exposure levels of abemaciclib. Beyond tolerability, the safety data were in line with the established characteristics of abemaciclib and findings from other vepdegestrant clinical trials. These results lend support to the continuing Phase 2 segment of the research, which is investigating the standard dose of abemaciclib (150 mg twice daily) in conjunction with vepdegestrant (200 mg daily) for patients with post-CDK4/6 advanced breast cancer.
"The early findings from this Phase 1b sub-study involving patients whose cancer had progressed following CDK4/6 inhibition are promising," stated Noah Berkowitz, M.D., Ph.D., Chief Medical Officer at Arvinas. "These results bolster our conviction that vepdegestrant may be effective in various combination therapies for metastatic breast cancer and holds the potential to establish itself as a leading ER therapy. We are excited to proceed with the Phase 2 portion of the study assessing the standard initial dose of abemaciclib alongside vepdegestrant."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 17, 2024, there are 289 investigational drugs for the ERα target, including 58 indications, 140 R&D institutions involved, with related clinical trials reaching 150, and as many as 21504 patents.
Vepdegestrant is a small molecule drug that falls into the category of Proteolysis-targeting chimeras (PROTAC). It is designed to target the ERα protein, making it a potential treatment option for various neoplasms, skin, and musculoskeletal diseases. The drug is specifically indicated for ER-positive/HER2-negative breast cancer, advanced breast cancer, estrogen receptor-positive breast cancer, and metastatic breast cancer.