Assembly Biosciences Administers First Participant in Phase 1a/b Trial for Herpes Treatment ABI-5366
Assembly Biosciences, Inc., a biotechnology firm focusing on the development of cutting-edge treatments for severe viral infections, has reported that the initial participant has received a dose in the Phase 1a/b clinical trial of its long-acting herpes simplex virus helicase-primase inhibitor, ABI-5366.
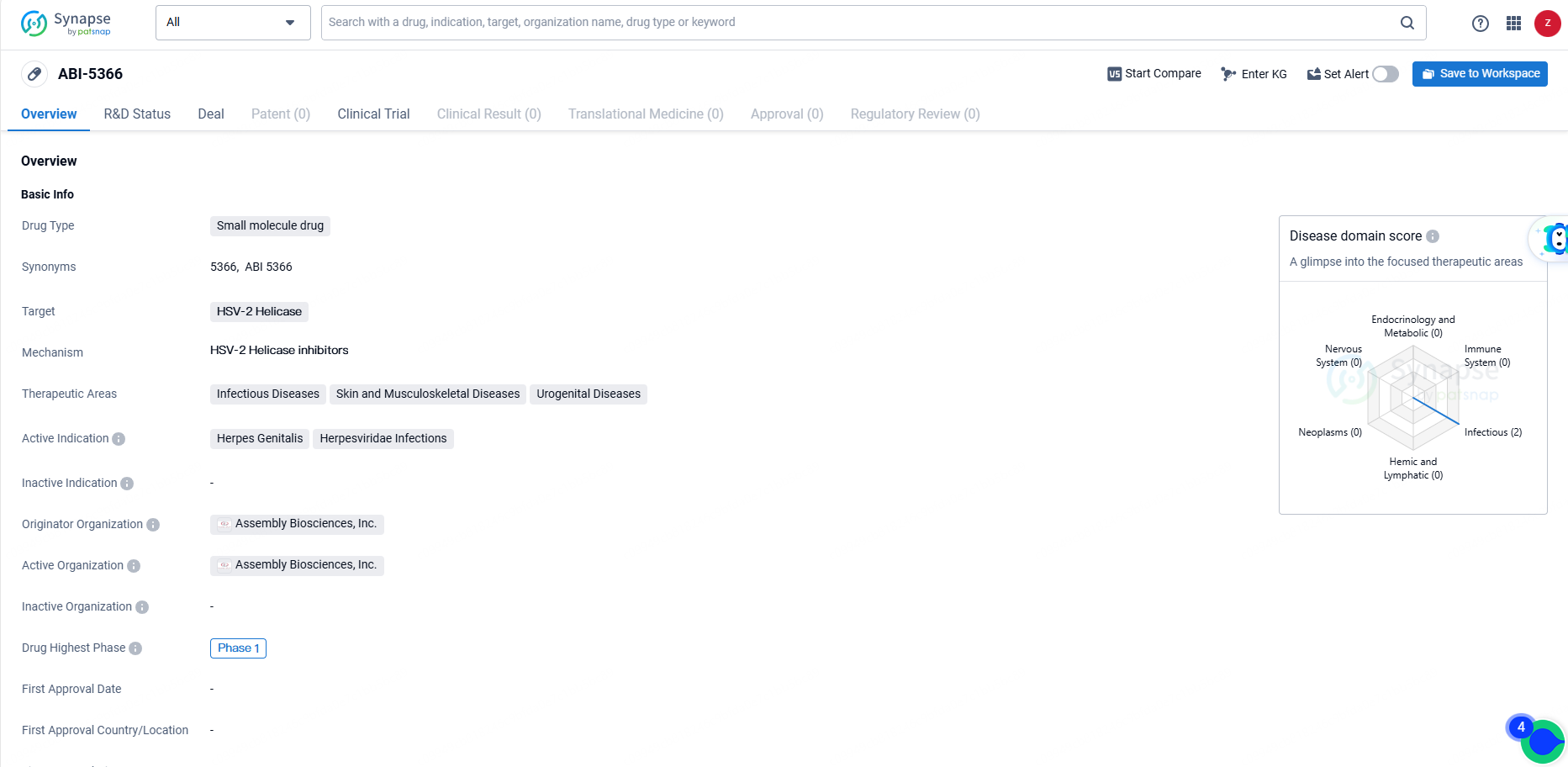
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ABI-5366, under development for treating recurrent genital herpes, has shown nanomolar efficacy against both HSV-1 and HSV-2 in vitro. Preclinical pharmacokinetic analyses suggest it could be suitable for extended-release administration. Recurrent genital herpes, due to HSV-1 or HSV-2, frequently occurs following an initial episode and affects approximately four million people in the U.S. and EU.
Current treatments are limited in efficacy, managing to control the infection or reducing transmission risk only partially even with daily chronic use. Among patients with recurrent genital herpes, only 34% remained free from recurrence after a year on current suppressive therapies.
“We are excited to progress ABI-5366 to clinical phases for recurrent genital herpes, a condition significantly impacting millions, with no new treatments approved in over 25 years,” commented Anuj Gaggar, MD, PhD, chief medical officer at Assembly Bio.
“Considering that HSV helicase-primase is a confirmed viral target, the pharmacokinetic data from healthy volunteers in Phase 1a will help us determine if ABI-5366 reaches the desired concentrations for antiviral efficiency and supports a weekly oral dose regimen. This will also guide our dose selection for the multiple ascending dose Phase 1b part of the trial, where viral and clinical effects in recurrent genital herpes patients will be assessed,” Gaggar added.
The ABI-5366-101 study is a randomized, blinded, placebo-controlled Phase 1a/b clinical trial. Part A has started dosing healthy participants to assess the safety, tolerability, and pharmacokinetics of ABI-5366 after single ascending doses. Subjects in Part A are randomized 6:2 between ABI-5366 and placebo across up to five dose cohorts.
Assembly Bio intends to proceed directly to Part B for HSV-2 seropositive individuals with recurrent genital herpes, aiming to begin by year-end. This phase will test multiple ascending doses of ABI-5366 with a 29-day weekly oral dosing regimen. Participants in Part B will be randomized 20:5 between ABI-5366 and placebo across four dose levels, with a pooled placebo analysis.
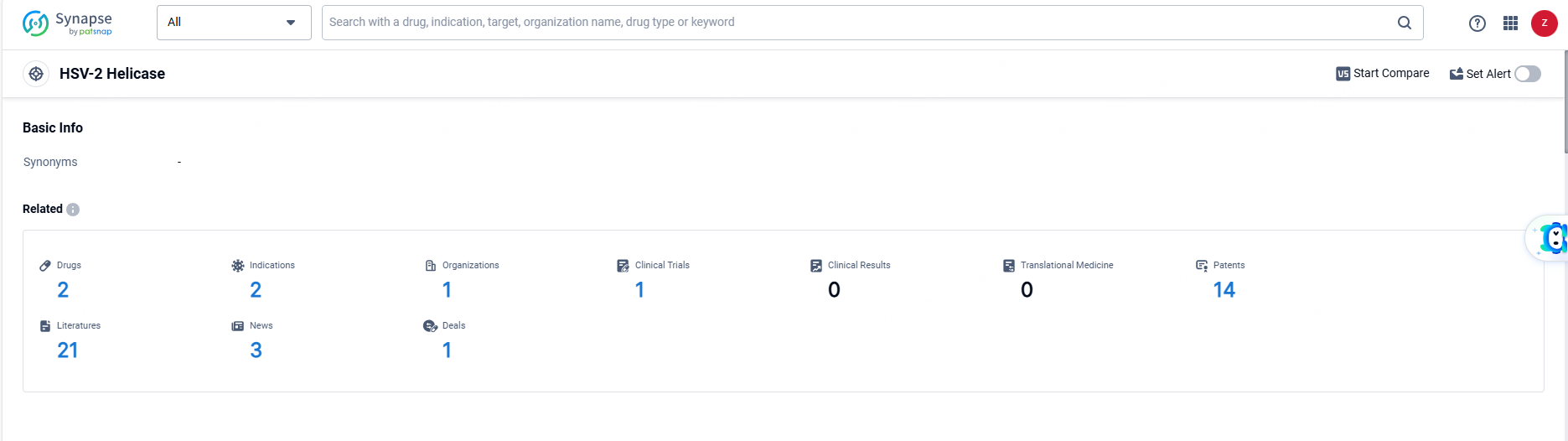
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 2 investigational drugs for the HSV-2 helicase target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 14 patents.
ABI-5366 is intended to address herpes genitalis and infections caused by the Herpesviridae family, with a focus on infectious and urogenital diseases. As of the latest available information, ABI-5366 has reached Phase 1 of clinical development, marking an important milestone in its journey towards potential approval and commercialization.