AstraZeneca Eyes Amolyt Pharma Acquisition to Expand Rare Disease Portfolio
AstraZeneca has declared the finalization of its plans to purchase Amolyt Pharma, a biotech entity in the clinical phase dedicated to creating innovative therapies for uncommon hormonal disorders.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The proposed acquisition will bolster the Alexion, AstraZeneca Rare Disease late-stage pipeline and
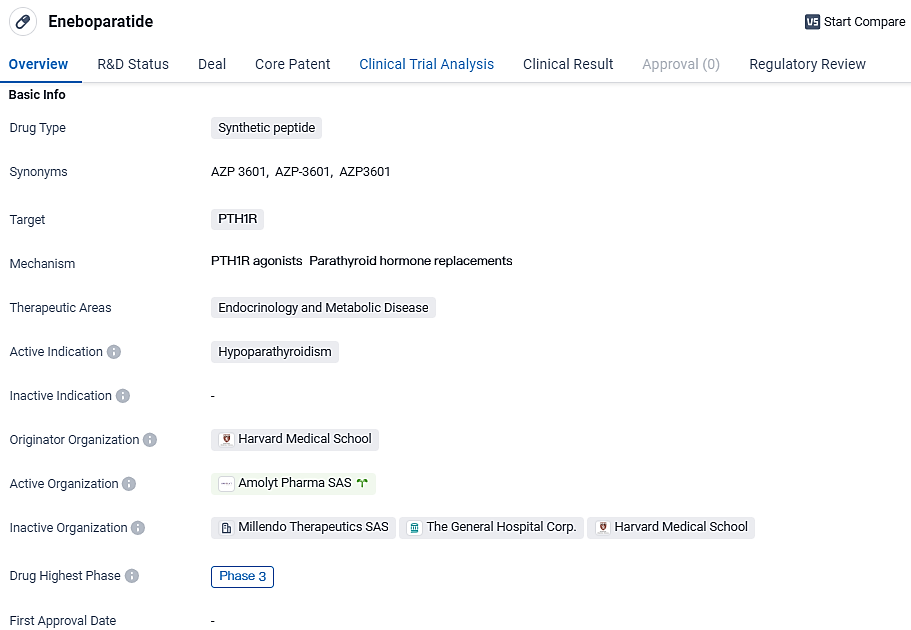
The strategic acquisition is poised to enhance Alexion's contributions to the AstraZeneca Rare Disease portfolio by broadening its bone health division. A pivotal addition is the incorporation of eneboparatide (AZP-3601), a Phase III peptide under investigation that presents an innovative approach aimed to address critical therapeutic needs in hypoparathyroidism treatment. Furthermore, Alexion eagerly anticipates the integration of skilled professionals from Amolyt Pharma.
As a forerunner in the rare disease sector, Alexion is exceptionally well-equipped to advance the late-phase development and achieve worldwide market release of eneboparatide. This therapeutic candidate promises to mitigate the severe consequences of reduced parathyroid hormone levels and circumvent the complications associated with excessive calcium supplement administration. We are confident that this initiative, augmented by the proficient team and specialized knowledge from Amolyt Pharma, as well as their preliminary drug pipeline, will facilitate our foray into the niche field of rare endocrine disorders.
Eneboparatide acts on the PTH receptor 1 (PTHR1) and has been ingeniously engineered to fulfill therapeutic objectives for those with hypoparathyroidism. According to Phase II clinical study outcomes, eneboparatide was successful in restoring normal serum calcium concentrations and showed promise in potentially eliminating the need for regular calcium and vitamin D supplementation.
In individuals experiencing chronic hypoparathyroidism accompanied by hypercalciuria, eneboparatide was effective in regulating urinary calcium levels. Furthermore, in hypoparathyroidism patients, the drug demonstrated its ability to maintain bone mineral density, a potentially significant advantage for patients at increased risk for conditions such as osteopenia or osteoporosis.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
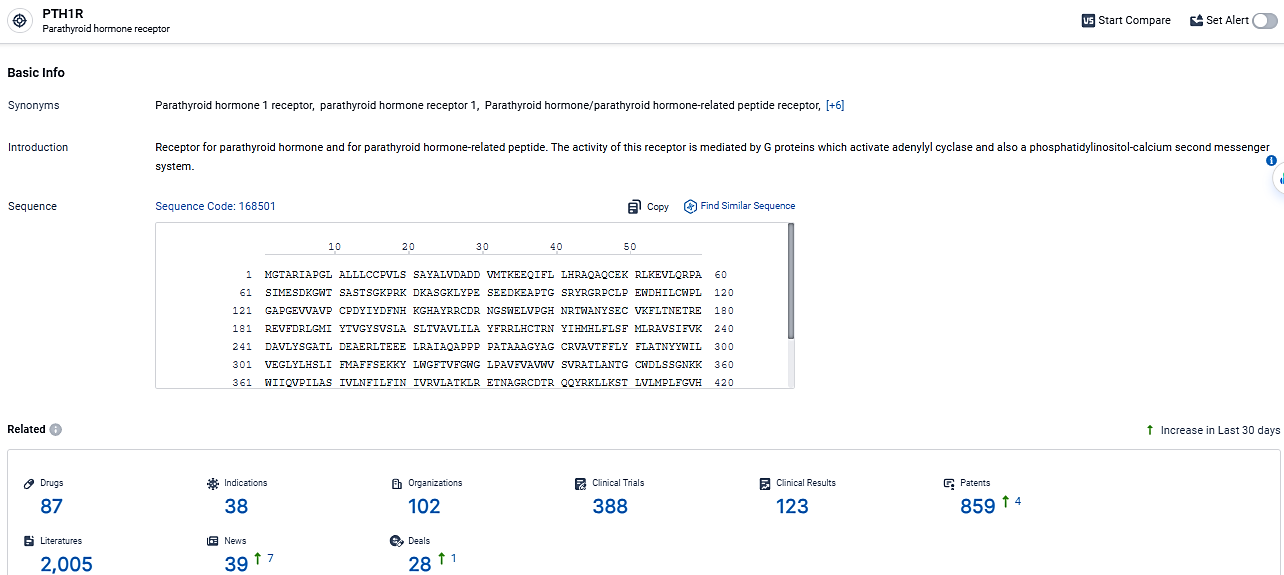
According to the data provided by the Synapse Database, As of March 18 2024, there are 87 investigational drugs for the PTHR1 target, including 38 indications, 102 R&D institutions involved, with related clinical trials reaching 388, and as many as 859 patents.
Eneboparatide shows promise as a potential treatment for hypoparathyroidism, a condition characterized by insufficient levels of parathyroid hormone. By targeting the PTH1R receptor, the drug aims to restore the balance of PTH in the body, potentially alleviating the symptoms associated with hypoparathyroidism.