AstraZeneca's C5 complement inhibitor, Eculizumab, has been approved in China for a new indication: the treatment of Neuromyelitis Optica Spectrum Disorder
Recently, AstraZeneca announced that the National Medical Products Administration (NMPA) of China has approved C5 complement inhibitor Eculizumab for the treatment of adults with anti-aquaporin-4 (AQP4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
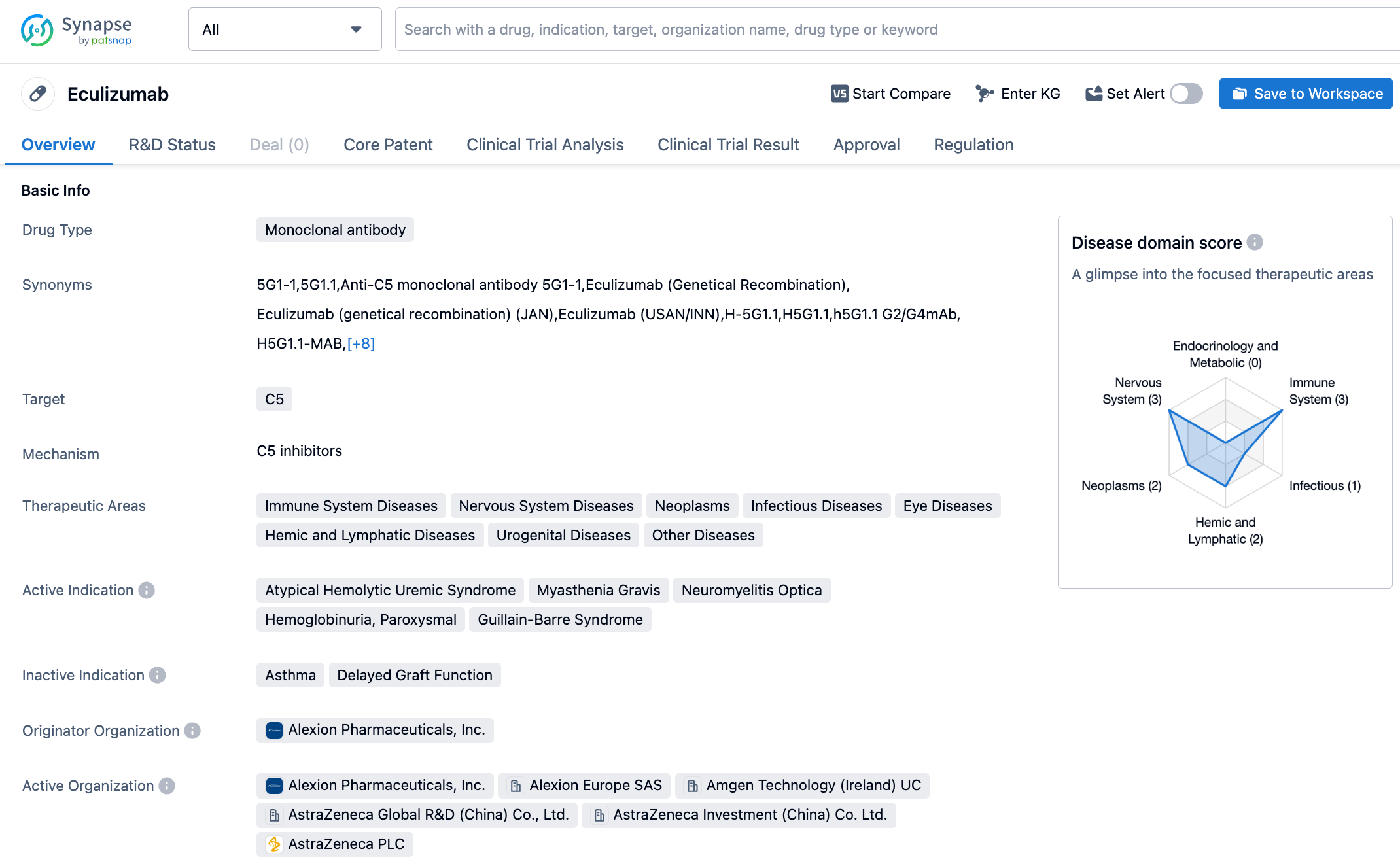
Eculizumab is a recombinant monoclonal antibody that can recognize and bind to antigens present in the human body. It attaches to the C5 complement protein and blocks it. The C5 complement protein is a part of the immune system's complement cascade reaction, and its activation can lead to the aforementioned condition. Soliris (eculizumab, or Eculizumab) is a drug used for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), generalized myasthenia gravis (gMG) and NMOSD. Eculizumab was originally developed by Alexion, and on December 12, 2020, AstraZeneca acquired it and another long-acting C5 complement inhibitor Ultomiris (Ravulizumab) when it purchased Alexion for $39 billion. According to AstraZeneca's financial report, the global sales of Eculizumab reached $3.762 billion in 2022.
The approval of Eculizumab in China for NMOSD is mainly based on the results of a pivotal phase 3 PREVENT study. The study showed that Eculizumab treatment significantly reduced relapse risk, with statistical and clinical significance. At week 48, 98% of patients treated with Eculizumab (compared to 63% of placebo-treated patients) had no relapses (a relative risk reduction of 94.2%), and this benefit persisted up to week 144. Additionally, in the 144-week PREVENT study, 96% of patients treated with Eculizumab had no relapses, while the no relapse rate was only 45% in placebo-treated patients. The safety and tolerability of Eculizumab were consistent with the results during the PREVENT study and its open-label extension studies. The most common adverse reactions during treatment were upper respiratory tract infection, headache, nasopharyngitis and nausea.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
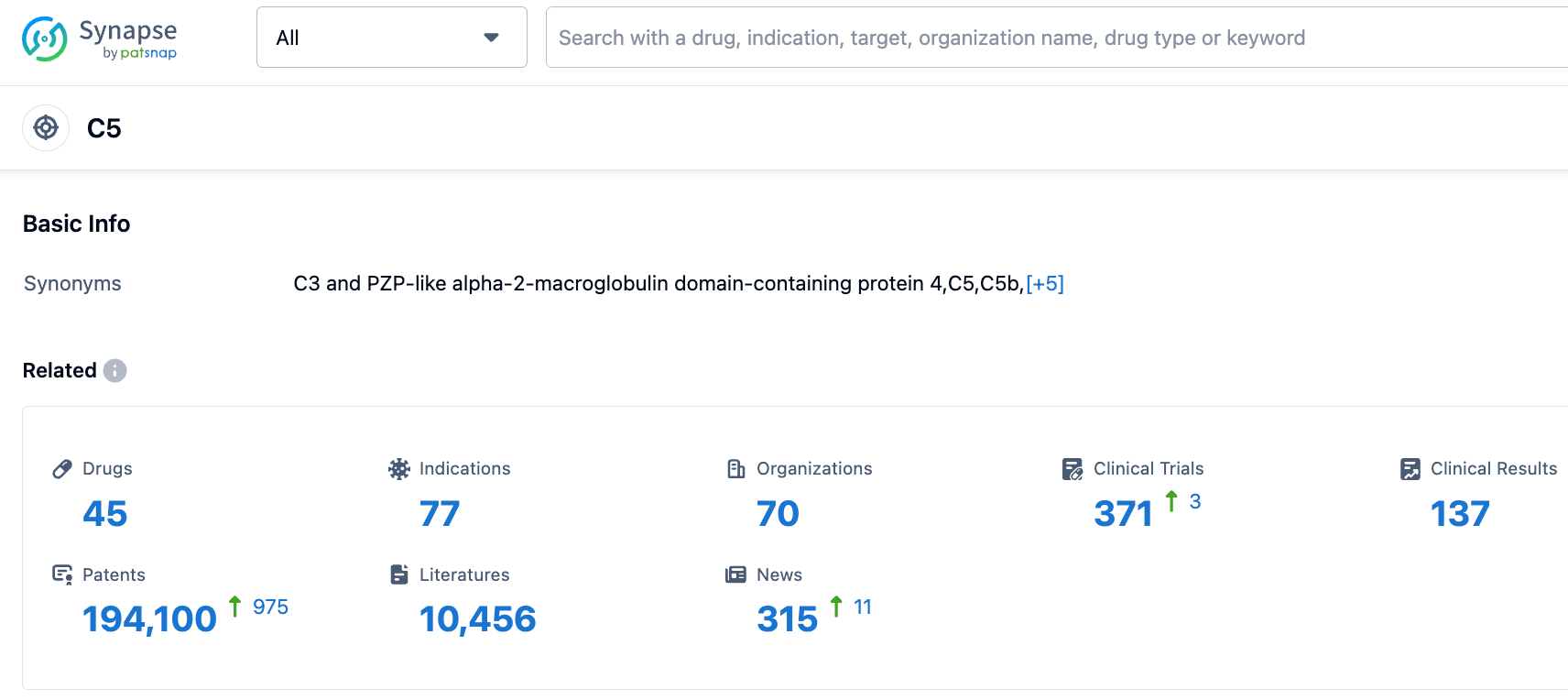
According to the Synapse database, as of October 20, 2023, there are 45 drugs under development targeting C5, with 77 indications, 70 research institutions involved, 371 related clinical trials, and as many as 193,768 patents. We look forward to the continued performance of Eculizumab.